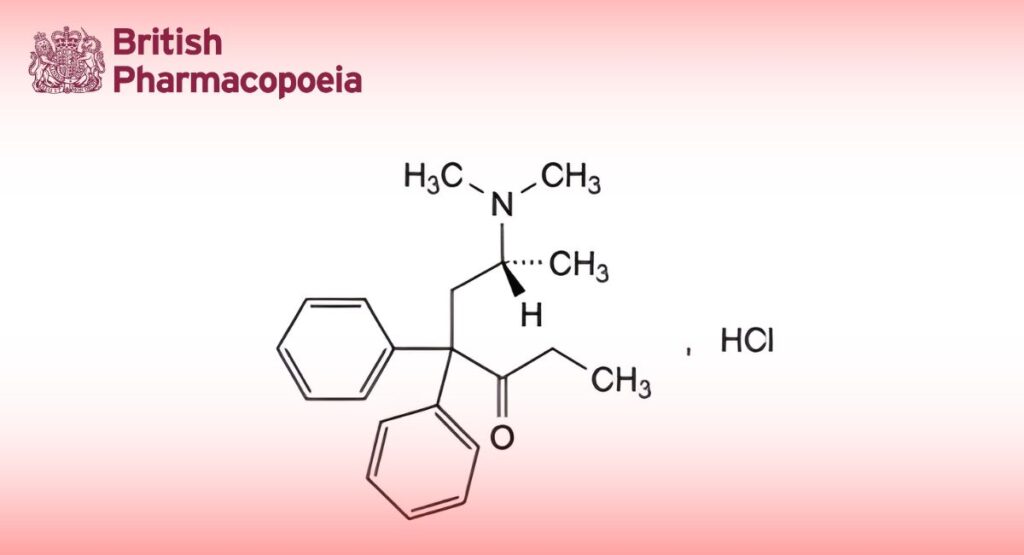
(Ph. Eur. monograph 1787)
C21H28ClNO 345.9 5967-73-7
Action and use
Opioid analgesic.
DEFINITION
(6R)-6-(Dimethylamino)-4,4-diphenylheptan-3-one hydrochloride.
Content
99.0 per cent to 101.0 per cent (dried substance).
CHARACTERS
Appearance
White or almost white, crystalline powder.
Solubility
Soluble in water, freely soluble in ethanol (96 per cent).
IDENTIFICATION
First identification: A, C, D.
Second identification: A, B, D.
A. Specific optical rotation (see Tests).
B. Melting point (2.2.14): 239 °C to 242 °C.
C. Infrared absorption spectrophotometry (2.2.24).
Comparison: Ph. Eur. reference spectrum of methadone hydrochloride.
D. Dilute 1 mL of solution S (see Tests) to 5 mL with water R and add 1 mL of dilute ammonia R1. Mix, allow to stand for 5 min and filter. The filtrate gives reaction (a) of chlorides (2.3.1).
TESTS
Solution S
Dissolve 2.50 g in carbon dioxide-free water R and dilute to 50.0 mL with the same solvent.
Appearance of solution
Solution S is clear (2.2.1) and colourless (2.2.2, Method II).
Acidity or alkalinity
Dilute 10 mL of solution S to 25 mL with carbon dioxide-free water R. To 10 mL of the solution add 0.2 mL of methyl red solution R and 0.2 mL of 0.01 M sodium hydroxide. The solution is yellow. Add 0.4 mL of 0.01 M hydrochloric acid. The solution is red.
Specific optical rotation (2.2.7)
-125 to -135 (dried substance), determined on solution S.
Related substances
Liquid chromatography (2.2.29).
Test solution: Dissolve 25.0 mg of the substance to be examined in the mobile phase and dilute to 100.0 mL with the mobile phase.
Reference solution (a): Dilute 1.0 mL of the test solution to 50.0 mL with the mobile phase. Dilute 1.0 mL of the solution to 10.0 mL with the mobile phase.
Reference solution (b): Dissolve 12.0 mg of imipramine hydrochloride CRS in the mobile phase and dilute to 10 mL with the mobile phase. To 1 mL of the solution add 5 mL of the test solution and dilute to 10 mL with the mobile phase.
Column:
— size: l = 0.125 m, Ø = 4.6 mm;
— stationary phase: octadecylsilyl silica gel for chromatography R (5 μm);
— temperature: 25 °C.
Mobile phase: Mix 35 volumes of acetonitrile R and 65 volumes of an 11.5 g/L solution of phosphoric acid R adjusted to pH 3.6 with tetraethylammonium hydroxide solution R.
Flow rate: 1.0 mL/min.
Detection: Spectrophotometer at 210 nm.
Equilibration: About 30 min.
Injection: 10 μL.
Run time: 7 times the retention time of levomethadone.
Retention time: Levomethadone = about 5 min.
System suitability: Reference solution (b):
— resolution: minimum 2.5 between the peaks due to imipramine and levomethadone.
Limits:
— any impurity: not more than 0.5 times the area of the principal peak in the chromatogram obtained with reference solution (a) (0.1 per cent);
— total: not more than 2.5 times the area of the principal peak in the chromatogram obtained with reference solution (a) (0.5 per cent);
— disregard limit: 0.25 times the area of the principal peak in the chromatogram obtained with reference solution (a) (0.05 per cent).
Dextromethadone
Liquid chromatography (2.2.29).
Test solution: Dissolve 40.0 mg of the substance to be examined in the mobile phase and dilute to 100.0 mL with the mobile phase.
Reference solution: Dilute 1.0 mL of the test solution to 10.0 mL with the mobile phase. Dilute 1.0 mL of this solution to 20.0 mL with the mobile phase.
Column:
— size: l = 0.25 m, Ø = 4.6 mm;
— stationary phase: 2-hydroxypropylbetadex for chromatography R (5 μm);
— temperature: 10 °C.
Mobile phase: Mix 1 volume of triethylamine R adjusted to pH 4.0 with phosphoric acid R, 15 volumes of acetonitrile R and 85 volumes of a 13.6 g/L solution of potassium dihydrogen phosphate R.
Flow rate: 0.7 mL/min.
Detection: Spectrophotometer at 210 nm.
Equilibration: About 30 min.
Injection: 10 μL.
Relative retention: With reference to levomethadone: dextromethadone = about 1.4.
System suitability: Test solution:
— number of theoretical plates: minimum 2000, calculated for the peak due to levomethadone;
— tailing factor: maximum 3 for the peak due to levomethadone.
Limit:
— dextromethadone: not more than the area of the principal peak in the chromatogram obtained with the reference solution (0.5 per cent).
Loss on drying (2.2.32)
Maximum 0.5 per cent, determined on 1.000 g by drying in an oven at 105 °C.
Sulfated ash (2.4.14)
Maximum 0.1 per cent, determined on 1.0 g.
ASSAY
Dissolve 0.300 g in a mixture of 40 mL of water R and 5 mL of acetic acid R. Titrate with 0.1 M silver nitrate. Determine the end-point potentiometrically (2.2.20), using a silver electrode.
1 mL of 0.1 M silver nitrate is equivalent to 34.59 mg of C21H28ClNO.
STORAGE
Protected from light.
IMPURITIES
Specified impurities A, B, C, D, E, F.
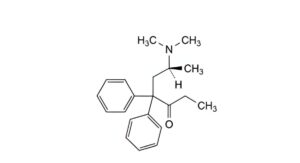
A. (6S)-6-(dimethylamino)-4,4-diphenylheptan-3-one,
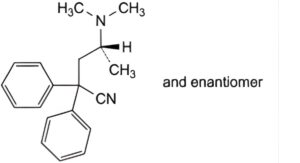
B. (4RS)-4-(dimethylamino)-2,2-diphenylpentanenitrile,
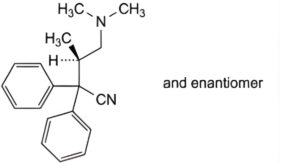
C. (3RS)-4-(dimethylamino)-3-methyl-2,2-diphenylbutanenitrile,
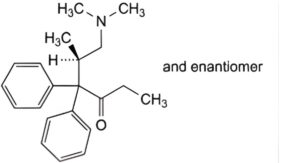
D. (5RS)-6-(dimethylamino)-5-methyl-4,4-diphenylhexan-3-one,
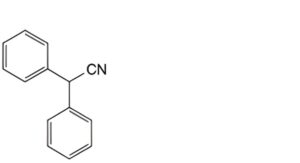
E. diphenylacetonitrile,
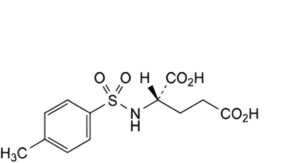
F. (2S)-2-[[(4-methylphenyl)sulfonyl]amino]pentanedioic acid (N-p-tosyl-L-glutamic acid).