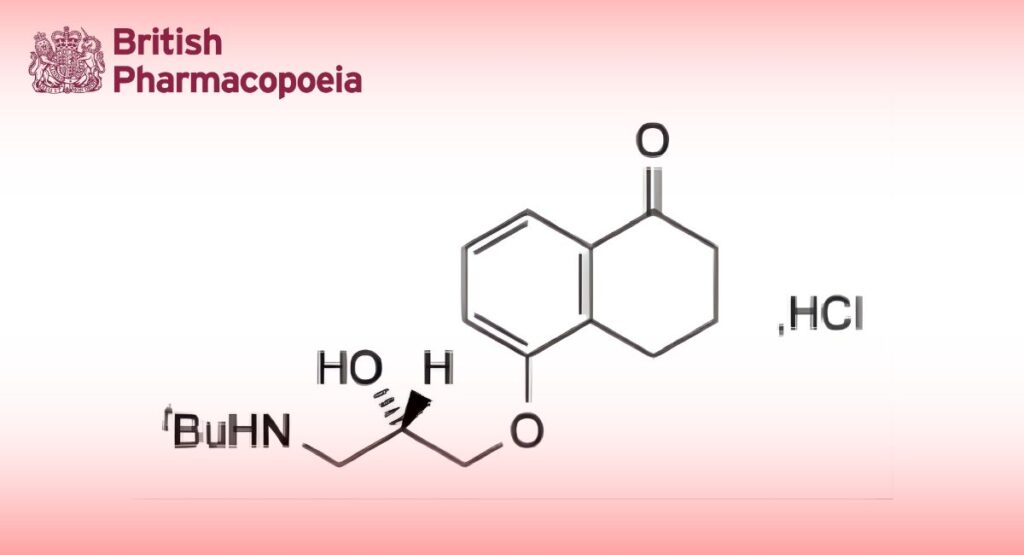
C17H25NO3,HCl 327.9 27912-14-7
Action and use
Beta-adrenoceptor antagonist.
Preparation
Levobunolol Eye Drops
DEFINITION
Levobunolol Hydrochloride is (S)-5-(3-tert-butylamino-2-hydroxypropoxy)-1,2,3,4-tetrahydronaphthalen-1-one hydrochloride. It contains not less than 98.5% and not more than 101.0% of C17H25NO3,HCl, calculated with reference to the dried substance.
CHARACTERISTICS
A white or pinkish white, crystalline powder.
Freely soluble in water; sparingly soluble in ethanol (96%).
IDENTIFICATION
A. The infrared absorption spectrum, Appendix II A, is concordant with the reference spectrum of levobunolol hydrochloride (RS 200).
B. Yields the reactions characteristic of chlorides, Appendix VI.
TESTS
Acidity
pH of a 5% w/v solution, 4.5 to 6.5, Appendix V L.
Specific optical rotation
In a 3% w/v solution in methanol, –19.0 to –20.0, calculated with reference to the dried substance, Appendix V F.
Related substances
Carry out the method for liquid chromatography, Appendix III D, using solutions in the mobile phase containing (1) 0.10% w/v of the substance being examined, (2) 0.00050% w/v of the substance being examined, (3) 0.0050% w/v of each of levobunolol hydrochloride BPCRS and atenolol.
The chromatographic procedure described under Assay may be used.
For solution (1) allow the chromatography to proceed for 3 times the retention time of the principal peak. The test is not valid unless, in the chromatogram obtained with solution (3), the resolution factor between the two principal peaks is at least 8.
In the chromatogram obtained with solution (1) the area of any secondary peak is not greater than the area of the principal peak in the chromatogram obtained with solution (2) (0.5%) and the sum of the areas of any secondary peaks is not greater than twice the area of the principal peak in the chromatogram obtained with
solution (2) (1%).
Loss on drying
When dried over phosphorus pentoxide at 110° at a pressure not exceeding 2 kPa for 4 hours, loses not more than 0.5% of its weight. Use 1 g.
ASSAY
Carry out the method for liquid chromatography, Appendix III D, using solutions in the mobile phase containing (1) 0.01% w/v of the substance being examined, (2) 0.01% w/v of levobunolol hydrochloride BPCRS and (3) 0.0050% w/v of each of levobunolol hydrochloride BPCRS and atenolol.
The chromatographic procedure may be carried out using (a) a stainless steel column (25 cm × 3.9 mm) packed with end-capped octylsilyl silica gel for chromatography (10 μm) (Lichrosorb RP 8 is suitable), (b) a solution prepared by mixing 53 volumes of 0.005M sodium heptanesulfonate in methanol with 47 volumes of 0.005M sodium heptanesulfonate in water containing 1 mL of 0.5M sulfuric acid as the mobile phase with a flow rate of 1 mL per minute and (c) a detection wavelength of 223 nm.
The test is not valid unless in the chromatogram obtained with solution (3) the resolution factor between the two principal peaks is at least 8.
Calculate the content of C17H25NO3,HCl from the declared content of C17H25NO3,HCl in levobunolol hydrochloride BPCRS.
STORAGE
Levobunolol Hydrochloride should be protected from light.
IMPURITIES
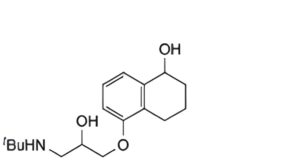
A. 5-(3-tert-butylamino-2-hydroxypropoxy)-1,2,3,4-tetrahydro-1-naphthol
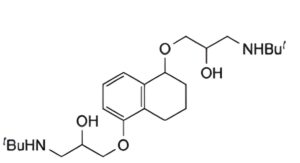
B. 1,1′-(1,2,3,4-tetrahydro-1,5-naphthalenedioxy)bis(3-tert-butylamino)-2-propanol
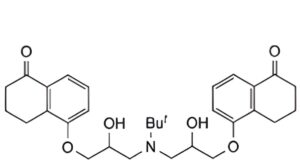
C. meso-5,5′-[(3,3′-tert-butylamino)bis(2-hydroxypropoxy)]bis-3,4-dihydronaphthalen-1(2 H)-one