Edition: BP 2025 (Ph. Eur. 11.6 update)
Action and use
Beta-adrenoceptor antagonist.
DEFINITION
Levobunolol Eye Drops are a sterile solution of Levobunolol Hydrochloride in Purified Water.
The eye drops comply with the requirements stated under Eye Preparations and with the following requirements.
Content of levobunolol hydrochloride, C17H25NO3,HCl
90.0 to 110.0% of the stated amount.
IDENTIFICATION
A. The light absorption, Appendix II B, in the range 210 to 350 nm of a solution prepared by diluting the eye drops with ethanol (96%) to contain 0.001% w/v of Levobunolol Hydrochloride exhibits two maxima, at 223 nm and at 255 nm and a broad peak at 315 nm.
B. In the Assay, the chromatogram obtained with solution (1) shows a peak with the same retention time as that of the principal peak in the chromatogram obtained with solution (2).
TESTS
Acidity or alkalinity
pH, 5.5 to 7.5, Appendix V L.
Related substances
The nominal total amount of related substances determined by tests A and B below is not more than 2.5% of the stated content of Levobunolol Hydrochloride.
A. Carry out the method for liquid chromatography, Appendix III D, using the following solutions.
(1) Dilute a suitable volume of the eye drops with the mobile phase to produce a solution containing 0.10% w/v of Levobunolol Hydrochloride.
(2) Dilute 1 volume of solution (1) to 100 volumes with the mobile phase.
(3) 0.0010% w/v of disodium edetate in the mobile phase.
CHROMATOGRAPHIC CONDITIONS
The chromatographic conditions described under Assay may be used.
LIMITS
In the chromatogram obtained with solution (1):
the area of any secondary peak is not greater than the area of the principal peak in the chromatogram obtained with solution (2) (1%).
Determine the sum of the areas of any secondary peaks.
Disregard any peak corresponding to the principal peak in the chromatogram obtained with solution (3).
B. Carry out test A as described above but using a detection wavelength of 400 nm and injecting solution (1).
LIMITS
The area of any peak with a retention time corresponding to that of the principal peak in the chromatogram obtained with solution (2) in test A is not greater than one-fifth of the area of that peak (1%, assuming a response factor of 5).
Calculate the nominal percentage content of this impurity from the area of the peak in the chromatogram obtained with solution (1) taking one-fifth of the area of the peak in the chromatogram obtained with solution (2) in test A to be equivalent to 1%.
ASSAY
Carry out the method for liquid chromatography, Appendix III D, using the following solutions.
(1) Dilute a suitable volume of the eye drops with the mobile phase to produce a solution containing 0.005% w/v of Levobunolol Hydrochloride.
(2) 0.005% w/v of levobunolol hydrochloride BPCRS in the mobile phase.
CHROMATOGRAPHIC CONDITIONS
(a) Use a stainless steel column (30 cm × 3.9 mm) packed with octadecylsilyl silica gel for chromatography (10 μm) (μBondapak C18 is suitable).
(b) Use isocratic elution and the mobile phase described below.
(c) Use a flow rate of 1.5 mL per minute.
(d) Use an ambient column temperature.
(e) Use a detection wavelength of 254 nm.
(f) Inject 20 μL of each solution.
MOBILE PHASE
5 volumes of glacial acetic acid, 450 volumes of 0.005M sodium heptanesulfonate and 550 volumes of methanol.
DETERMINATION OF CONTENT
Calculate the content of C17H25NO3,HCl in the eye drops from the declared content of C17H25NO3,HCl in levobunolol hydrochloride BPCRS.
STORAGE
Levobunolol Eye Drops should be protected from light.
IMPURITIES
The impurities limited by the requirements of this monograph include:
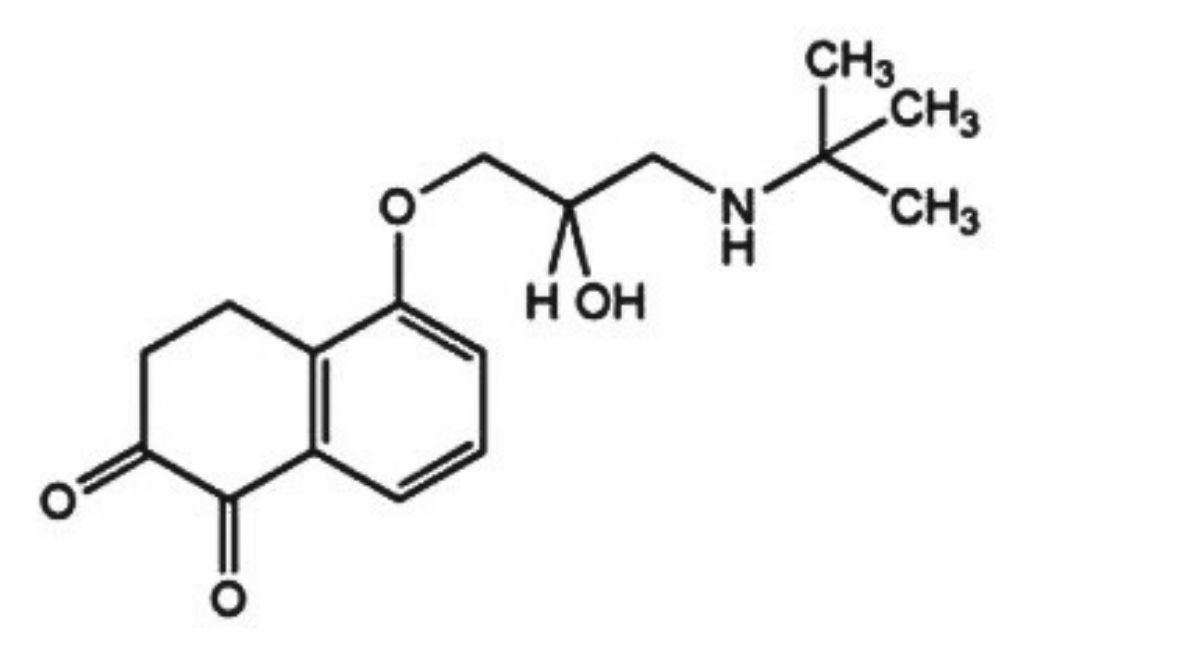
A. 5-(3-tert-butylamino-2-hydroxypropoxy)-3,4-dihydro-1,2-naphthalene dione,
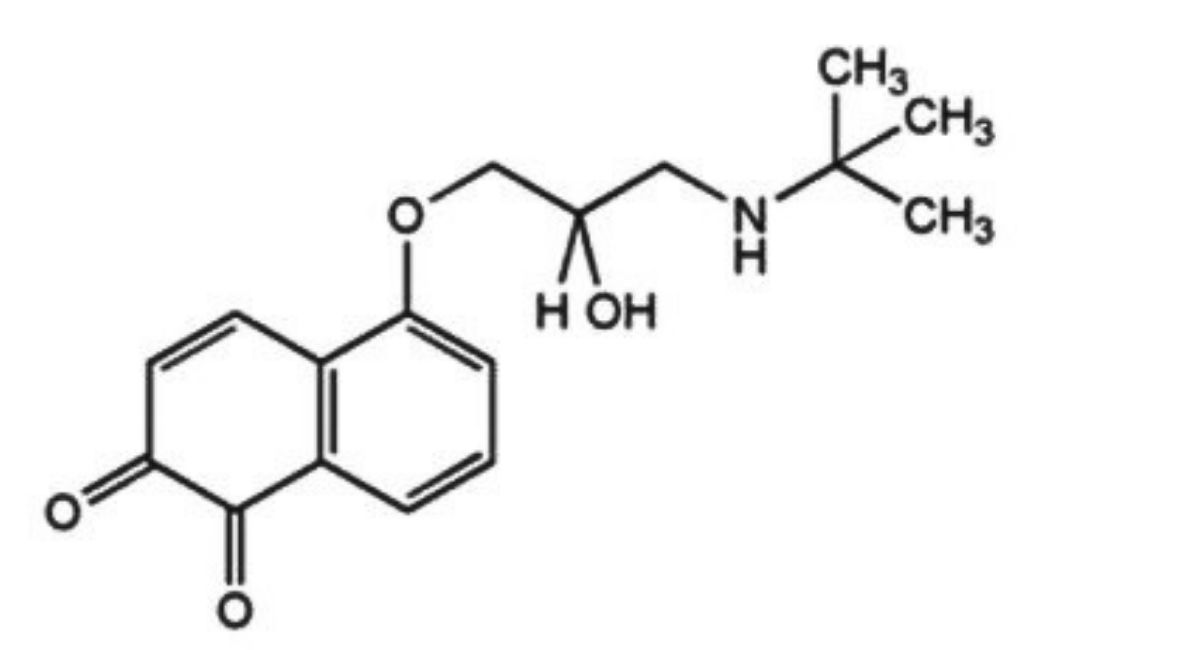
B. 5-(3-tert-butylamino-2-hydroxypropoxy)-1,2-naphthoquinone.






