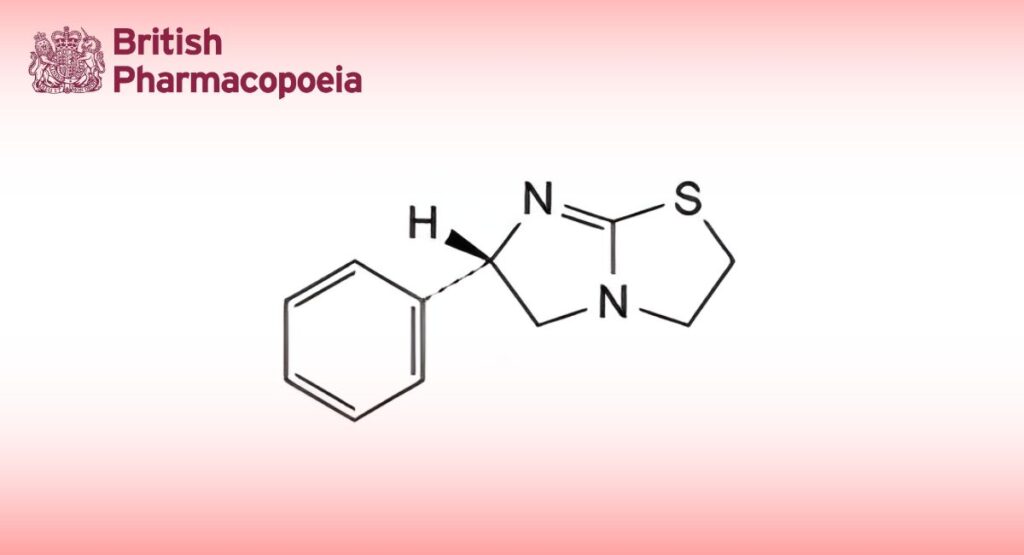
(Levamisole for Veterinary Use, Ph. Eur. monograph 1728)
C11H12N2S 204.3 14769-73-4
Action and use
Immunostimulant; antihelminthic.
DEFINITION
(6S)-6-Phenyl-2,3,5,6-tetrahydroimidazo[2,1-b]thiazole.
Content
98.5 per cent to 101.5 per cent (anhydrous substance).
CHARACTERS
Appearance
White or almost white powder.
Solubility
Slightly soluble in water, freely soluble in ethanol (96 per cent) and in methanol.
It shows polymorphism (5.9).
IDENTIFICATION
A. Specific optical rotation (see Tests).
B. Infrared absorption spectrophotometry (2.2.24).
Comparison: Ph. Eur. reference spectrum of levamisole.
If the spectra show differences, dissolve the substance to be examined in methylene chloride R, evaporate to dryness and record a new spectrum using the residue.
TESTS
Solution S
Dissolve 2.50 g in ethanol R and dilute to 50.0 mL with the same solvent.
Appearance of solution
Solution S is clear (2.2.1) and not more intensely coloured than reference solution BY6 (2.2.2, Method II).
Specific optical rotation (2.2.7)
-89 to -85 (anhydrous substance), determined on solution S.
Related substances
Liquid chromatography (2.2.29). Prepare the solutions immediately before use, protect from light and keep below 25 °C.
Test solution: Dissolve 0.100 g of the substance to be examined in methanol R and dilute to 10.0 mL with the same solvent.
Reference solution (a): Dissolve 10 mg of levamisole hydrochloride for system suitability CRS (containing impurities A, B, C, D and E) in methanol R, add 0.1 mL of concentrated ammonia R and dilute to 1.0 mL with methanol R.
Reference solution (b): Dilute 1.0 mL of the test solution to 100.0 mL with methanol R. Dilute 5.0 mL of the solution to 25.0 mL with methanol R.
Column:
— size: l = 0.10 m, Ø = 4.6 mm,
— stationary phase: base-deactivated octadecylsilyl silica gel for chromatography R (3 μm).
Mobile phase:
— mobile phase A: dissolve 0.5 g of ammonium dihydrogen phosphate R in 90 mL of water R; adjust to pH 6.5 with a 40 g/L solution of sodium hydroxide R and dilute to 100 mL with water R;
— mobile phase B: acetonitrile R;
| Time (min) |
Mobile phase A (per cent V/V) |
Mobile phase B (per cent V/V) |
| 0 – 8 | 90 → 30 | 10 → 70 |
| 8 – 10 | 30 | 70 |
Flow rate: 1.5 mL/min.
Detection: Spectrophotometer at 215 nm.
Injection: 10 μL.
Identification of impurities: Use the chromatogram supplied with levamisole hydrochloride for system suitability CRS and the chromatogram obtained with reference solution (a) to identify the peaks due to impurities A, B, C, D and E.
Relative retention: With reference to levamisole (retention time = about 3 min): impurity A = about 0.9; impurity B = about 1.4; impurity C = about 1.5; impurity D = about 1.6; impurity E = about 2.0.
System suitability: Reference solution (a):
— the chromatogram obtained is similar to the chromatogram supplied with levamisole hydrochloride for system suitability CRS.
Limits:
— correction factors: for the calculation of content, multiply the peak areas of the following impurities by the corresponding correction factor: impurity A = 2.0; impurity B = 1.7; impurity C = 2.9; impurity D = 1.3; impurity E = 2.7;
— impurities A, B, C, D, E: for each impurity, not more than the area of the principal peak in the chromatogram obtained with reference solution (b) (0.2 per cent);
— any other impurity: not more than half the area of the principal peak in the chromatogram obtained with reference solution (b) (0.1 per cent);
— total: not more than 1.5 times the area of the principal peak in the chromatogram obtained with reference solution (b) (0.3 per cent);
— disregard limit: 0.25 times the area of the principal peak in the chromatogram obtained with reference solution (b) (0.05 per cent).
Water (2.5.12)
Maximum 0.5 per cent, determined on 1.00 g.
Sulfated ash (2.4.14)
Maximum 0.1 per cent, determined on 1.0 g.
ASSAY
Dissolve 0.150 g in 50 mL of a mixture of 1 volume of anhydrous acetic acid R and 7 volumes of methyl ethyl ketone R. Titrate with 0.1 M perchloric acid, using 0.2 mL of naphtholbenzein solution R as indicator.
1 mL of 0.1 M perchloric acid is equivalent to 20.43 mg of C11H12N2S.
STORAGE
In an airtight container, protected from light.
IMPURITIES
Specified impurities A, B, C, D, E.
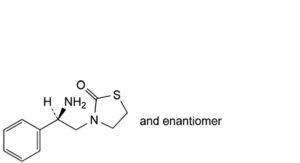
A. 3-[(2RS)-2-amino-2-phenylethyl]thiazolidin-2-one,
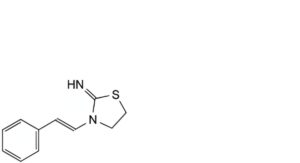
B. 3-[(E)-2-phenylethenyl]thiazolidin-2-imine,
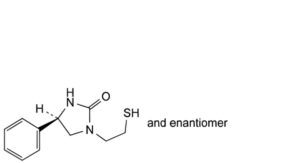
C. (4RS)-4-phenyl-1-(2-sulfanylethyl)imidazolidin-2-one,
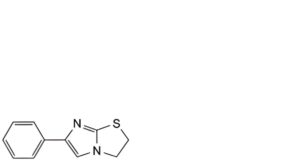
D. 6-phenyl-2,3-dihydroimidazo[2,1-b]thiazole,
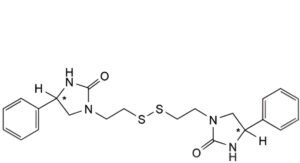
E. 1,1′-[(disulfane-1,2-diyl)bis(ethylene)]bis[(4RS)-4-phenylimidazolidin-2-one].