(Ph. Eur. monograph 2046)
Action and use
Non-ionic surfactant; sclerosant.
DEFINITION
Mixture of lauryl alcohol (dodecanol) monoethers of mixed macrogols. It may contain some free macrogols and it contains various amounts of free lauryl alcohol. The number of moles of ethylene oxide reacted per mole of lauryl alcohol is 9. The name of the substance is followed by a number (400) corresponding approximately to the average molecular mass of the macrogol portion.
This monograph applies to lauromacrogol 400 used as active substance.
CHARACTERS
Appearance
White or almost white, unctuous and hygroscopic mass, melting at 24 °C into a colourless or yellowish, viscous liquid.
Solubility
Freely soluble in water, very soluble in acetone and in ethanol (96 per cent).
IDENTIFICATION
A. Hydroxyl value (see Tests).
B. Saponification value (see Tests).
C. Warm the substance to be examined in an incubator at 50 °C for 1 h until fully molten and clear.
Transfer 50 mL to a warmed cloud-point tube (flat-bottomed glass tube 30-33.5 mm in internal diameter and 115-125 mm high). Insert the tube into a cooling bath that allows the outer surface of the tube to be in contact with chilled air, contained within a cylindrical metal container (internal diameter 9.5-12.5 mm greater than the external diameter of the sample tube, 115 mm high) that is surrounded by iced water. The base of the glass tube rests on a 6 mm thick cork disc, which prevents direct thermal contact with the cooled metal cylinder. Stir the substance to be examined continuously with a thermometer so that the temperature is constant throughout the substance. Periodically lift the tube out of the cooling bath to check for signs of cloudiness at the bottom of the tube. Examine the tube against a bright light source. When cloudiness is first observed, check more frequently until the substance becomes completely cloudy and the thermometer, suspended in the centre of the substance, is only just visible when viewed horizontally. Record the temperature. It is 20 °C to 25 °C.
TESTS
Appearance
The molten substance to be examined is clear (2.2.1) and not more intensely coloured than reference solution GY6 (2.2.2, Method I).
Alkalinity
Dissolve 2.0 g in a hot mixture of 10 mL of carbon dioxide-free water R and 10 mL of ethanol (96 per cent) R. Add 0.1 mL of bromothymol blue solution R1. Not more than 0.5 mL of 0.1 M hydrochloric acid is required to change the colour of the indicator to yellow.
Acid value (2.5.1)
Maximum 1.0, determined on 5.0 g.
Hydroxyl value (2.5.3, Method A)
90 to 105, determined on 2.0 g.
Iodine value (2.5.4, Method A)
Maximum 2.0.
Peroxide value
Maximum 5.0.
Introduce 10.0 g into a 100 mL beaker, dissolve with glacial acetic acid R and dilute to 20 mL with the same solvent. Add 1 mL of saturated potassium iodide solution R, mix and allow to stand for 1 min. Add 50 mL of carbon dioxide-free water R. Titrate with 0.01 M sodium thiosulfate, determining the end-point potentiometrically (2.2.20). Carry out a blank titration.
Determine the peroxide value using the following expression:
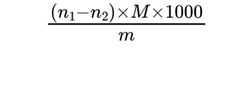
n1 = volume of 0.01 M sodium thiosulfate required for the substance to be examined, in millilitres;
n2 = volume of 0.01 M sodium thiosulfate required for the blank titration, in millilitres;
M = molarity of the sodium thiosulfate solution, in moles per litre;
m = mass of the substance to be examined, in grams.
Saponification value (2.5.6)
Maximum 3.0.
Free lauryl alcohol (dodecanol)
Gas chromatography (2.2.28).
Test solution: Dissolve 0.200 g of the substance to be examined in acetone R and dilute to 10.0 mL with the same solvent.
Reference solution: Dissolve 2.00 g of lauryl alcohol R in acetone R and dilute to 100.0 mL with the same solvent. Dilute 1.0 mL of the solution to 50.0 mL with acetone R.
Column:
— material: fused silica;
— size: l = 30 m, Ø = 0.25 mm;
— stationary phase: phenyl(5)methyl(95)polysiloxane R (film thickness 0.1 μm).
Carrier gas helium for chromatography R.
Flow rate: 1 mL/min.
Split ratio: 50:1.
Temperature:
| Time (min) | Temperature (°C) | |
| Column | 0 – 1 | 120 |
| 1 – 23 | 120 → 35 | |
| 23 – 33 | 350 | |
| Injection port | 300 | |
| Detector | 350 |
Detection: Flame ionisation.
Injection: 1.0 μL.
Retention time: Lauryl alcohol = about 5 min.
Limit:
— free lauryl alcohol: not more than the area of the corresponding peak in the chromatogram obtained with the reference solution (2.0 per cent).
Free macrogols
Size-exclusion chromatography (2.2.30).
Test solution: Dissolve 5.0 g of the substance to be examined in the mobile phase and dilute to 250.0 mL with the mobile phase.
Reference solution (a): Dissolve about 0.4 g of macrogol 1000 R in the mobile phase and dilute to 250.0 mL with the mobile phase.
Reference solution (b): Dilute 50.0 mL of reference solution (a) to 100.0 mL with the mobile phase.
Precolumns (2):
— size: l = 0.125 m, Ø = 4 mm;
— stationary phase: spherical octadecylsilyl silica gel for chromatography R (5 μm) with a pore size of 10 nm.
Column:
— size: l = 0.30 m, Ø = 7.8 mm;
— stationary phase: hydroxylated polymethacrylate gel R (6 μm) with a pore size of 12 nm.
Connect both precolumns to the column using a 3-way valve and switch the mobile phase flow according to the following programme:
— 0-114 s: precolumn 1 and column;
— 115 s to the end: precolumn 2 and column;
— 115 s to 8 min: flow back of precolumn 1.
Mobile phase: water R, methanol R (2:8 V/V).
Flow rate: 1.1 mL/min.
Detection: Refractometer.
Injection: 20 μL.
Calculate the percentage content of free macrogols using the following expression:
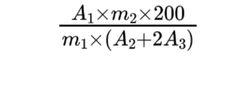
m1 = mass of the substance to be examined in the test solution, in grams;
m2 = mass of macrogol 1000 R in reference solution (a), in grams;
A1 = area of the peak due to free macrogols in the chromatogram obtained with the test solution;
A2 = area of the peak due to macrogol 1000 in the chromatogram obtained with reference solution (a);
A3 = area of the peak due to macrogol 1000 in the chromatogram obtained with reference solution (b).
Limit:
— free macrogols: maximum 3.0 per cent.
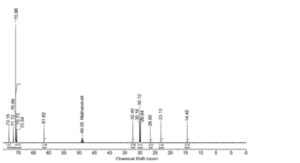
Figure 2046.-1. – 13C NMR spectrum of lauromacrogol 400
Average chain length of the fatty alcohol and average number of moles of ethylene oxide
Nuclear magnetic resonance spectrometry (2.2.33).
Test solution: If the substance is in the solid state at room temperature, heat gently before sampling.
Dissolve 0.4 mL of the substance to be examined in 0.3 mL of a mixture of 1 volume of deuterated methanol R and 2 volumes of deuterated chloroform R, containing 0.1 mol/L of chromium(III) acetylacetonate R as a relaxation aid.
Apparatus: High resolution FT-NMR spectrometer operating at minimum 300 MHz.
Acquisition of 13C NMR spectra The following parameters may be used:
— sweep width: 250 ppm (-15 ppm to 235 ppm);
— irradiation frequency offset: 110 ppm;
— time domain: 64 K;
— pulse delay: 3 s;
— pulse program: zgig 30 (inverse gated, 30° excitation pulse);
— dummy scans: 4;
— number of scans: 2048.
Processing and plotting: The following parameters may be used:
— size: 64 K (zero-filling);
— window multiplication: exponential;
— Lorentzian broadening factor: 1 Hz.
Use the CD3OD signal for shift referencing. The shift of the central peak of the multiplet is set to 49.0 ppm.
Plot the spectral region δ 0.0-80.0 ppm. Compare the spectrum with the spectrum in Figure 2046.-1. The shift values lie near the values given in Table 2046.-1.
Table 2046.-1. – Shift values
| Signal | Shift (ppm) | Normalised integrals |
| CH3 | 14.4 | 0.989 |
| CH2 (alkyl chain) | 23.2 | 1.000 |
| CH2 (alkyl chain) | 25.5 | 1.001 |
| CH2‘s (alkyl chain) | 30 | 7.410 |
| CH2 (alkyl chain) | 32.5 | 0.963 |
|
CH2 (-CH2-OH) (end CH2-group of macrogol)
|
61.6 | 1.001 |
| CH2‘s (macrogol) | 70.7 | 16.25 |
|
CH2 (R-CH-O-macrogol) (CH2 in alpha position)
|
72.6 | 0.998 |
| CH2 (macrogol) | 73.1 | 0.929 |
System suitability:
— signal-to-noise ratio: minimum 150, for the smallest relevant peak (CH2 at 73.1 ppm);
— peak width at half-height: maximum 0.05 ppm, for the central CDCl3 signal (at δ 78.6 ppm).
Calculation of the average chain length of the fatty alcohol and the average number of moles of ethylene oxide Define the signal at 23.2 ppm as 1.000 and normalise the integrals of the other signals listed in Table 2046.-1.
The average chain length of the fatty alcohol is calculated using the following expression:
∑14-33 In,i + In,72.6
∑14-33 In,i = sum of the normalised integrals of the signals from 14 ppm to 33 ppm;
In,72.6 = normalised integral of the signal at 72.6 ppm.
The average number of moles of ethylene oxide is calculated using the following expression:
0.5 x (In,62 + In,71 + In,73 )
In,62, In,71, In,73 = normalised integral of the signals at 62 ppm, 71 ppm and 73 ppm respectively.
The sum of the normalised integrals of the signals at 62 ppm, 71 ppm and 73 ppm corresponds to the average number of methylene groups in the macrogol part of lauromacrogol 400.
Limits:
— average chain length of the fatty alcohol: 10.0 to 14.0;
— average number of moles of ethylene oxide: 7.0 to 11.0.
Ethylene oxide and dioxan (2.4.25, Method A)
Maximum 1 ppm of ethylene oxide and maximum 10 ppm of dioxan.
Water (2.5.12)
Maximum 2.0 per cent, determined on 0.500 g.
Total ash (2.4.16)
Maximum 0.2 per cent, determined on 2.0 g.






