(Ph. Eur. monograph 0187)
NOTE: The name Lactose was formerly used in the United Kingdom.
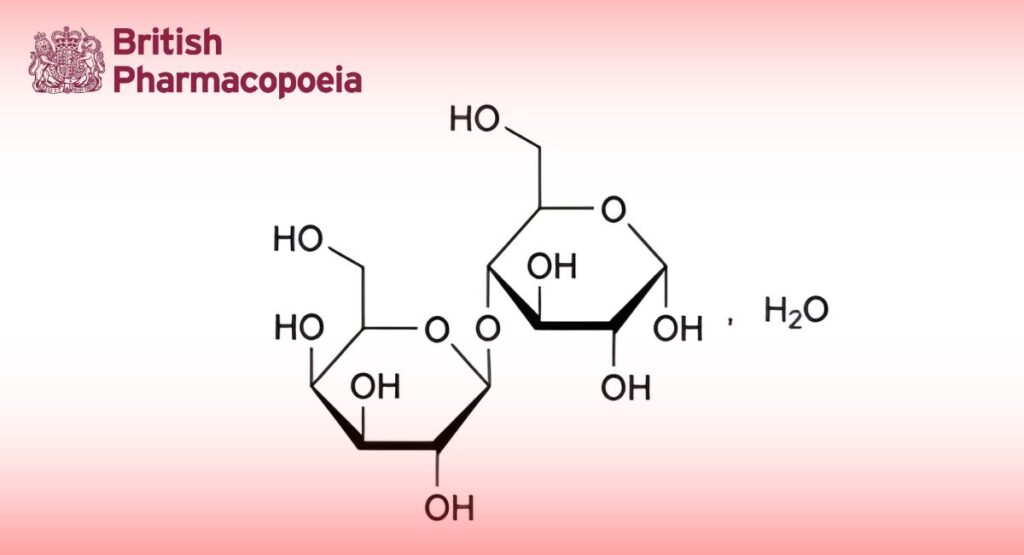
C12H22O11,H2O 360.3
Action and use
Excipient.
DEFINITION
O-β-D-Galactopyranosyl-(1→4)-α-D-glucopyranose monohydrate.
♦ CHARACTERS
Appearance
White or almost white, crystalline powder.
Solubility
Freely soluble in water, practically insoluble in ethanol (96 per cent).♦
IDENTIFICATION
First identification: A, ♢ D.
Second identification: B, C, D.♢
A. Infrared absorption spectrophotometry (2.2.24).
Comparison: lactose monohydrate CRS.
♢ B. Thin-layer chromatography (2.2.27).
Solvent mixture: water R, methanol R (40:60 V/V).
Test solution: Dissolve 10 mg of the substance to be examined in the solvent mixture and dilute to 20 mL with the solvent mixture.
Reference solution: Dissolve 10 mg of lactose monohydrate CRS in the solvent mixture and dilute to 20 mL with the solvent mixture.
Plate: TLC silica gel plate R.
Mobile phase: water R, methanol R, glacial acetic acid R, methylene chloride R (10:15:25:50 V/V/V/V); measure the volumes accurately, as a slight excess of water produces cloudiness.
Application: 2 μL; thoroughly dry the points of application.
Development: A Over 3/4 of the plate.
Drying A: In a current of warm air.
Development B: Immediately, over 3/4 of the plate, after renewing the mobile phase.
Drying B: In a current of warm air.
Detection: Spray with a solution of 0.5 g of thymol R in a mixture of 5 mL of sulfuric acid R and 95 mL of ethanol (96 per cent) R; heat at 130 °C for 10 min.
Results: The principal spot in the chromatogram obtained with the test solution is similar in position, colour and size to the principal spot in the chromatogram obtained with the reference solution.
C. Dissolve 0.25 g in 5 mL of water R. Add 5 mL of ammonia R and heat in a water-bath at 80 °C for 10 min. A red colour develops.
D. Water (see Tests).♢
TESTS
Solution S
Dissolve 1.0 g in boiling water R, allow to cool and dilute to 10.0 mL with water R.
Appearance of solution
Solution S is clear (2.2.1) ♢ and not more intensely coloured than reference solution BY7 (2.2.2, Method II)♢ .
Acidity or alkalinity
Dissolve 6.0 g by heating in 25 mL of carbon dioxide-free water R, cool and add 0.3 mL of phenolphthalein solution R1. The solution is colourless. Not more than 0.4 mL of 0.1 M sodium hydroxide is required to change the colour of the indicator to pink or red.
Specific optical rotation (2.2.7)
+ 54.4 to + 55.9 (anhydrous substance).
Dissolve 10.0 g in 80 mL of water R with heating at 50 °C. Allow to cool and add 0.2 mL of dilute ammonia R1. Allow to stand for 30 min and dilute to 100.0 mL with water R.
Absorbance: proteins and light-absorbing impurities (2.2.25)
Test solution (a): Solution S.
Test solution (b): Dilute 1.0 mL of test solution (a) to 10.0 mL with water R.
Spectral range: 400 nm for test solution (a) and 210-300 nm for test solution (b).
Results:
— at 400 nm: maximum 0.04 for test solution (a);
— from 210 nm to 220 nm: maximum 0.25 for test solution (b);
— from 270 nm to 300 nm: maximum 0.07 for test solution (b).
Water (2.5.12)
4.5 per cent to 5.5 per cent, determined on 0.50 g, using a mixture of 1 volume of formamide R and 2 volumes of methanol R as solvent.
Sulfated ash (2.4.14)
Maximum 0.1 per cent, determined on 1.0 g.
Microbial contamination
TAMC: acceptance criterion 102 CFU/g (2.6.12).
Absence of Escherichia coli (2.6.13).
♢ FUNCTIONALITY-RELATED CHARACTERISTICS
This section provides information on characteristics that are recognised as being relevant control parameters for one or more functions of the substance when used as an excipient (see chapter 5.15). Some of the characteristics described in the
Functionality-related characteristics section may also be present in the
mandatory part of the monograph since they also represent mandatory quality criteria. In such cases, a cross-reference to the tests described in the mandatory part is included in the Functionality-related characteristics section. Control of the characteristics can contribute to the quality of a medicinal product by improving the consistency of the manufacturing process and the performance of the medicinal product during use. Where control methods are cited, they are recognised as being suitable for the purpose, but other methods can also be used. Wherever results for a particular characteristic are reported, the control method must be indicated.
The following characteristics may be relevant for lactose monohydrate used as a filler/diluent in solid dosage forms (compressed and powder).
Particle size distribution (2.9.31 or 2.9.38)
Bulk density of powders (2.9.34)♢
(1) This monograph has undergone pharmacopoeial harmonisation. See chapter 5.8 Pharmacopoeial harmonisation.