Lactose1
Anhydrous Lactose
(Ph. Eur. monograph 1061)
C12H22O11 342.3 63-42-3
Action and use
Excipient.
DEFINITION
O-β-D-Galactopyranosyl-(1→4)-β-D-glucopyranose or mixture of O-β-D-galactopyranosyl-(1→4)-α-D-glucopyranose and O-β-D-galactopyranosyl-(1→4)-β-D-glucopyranose.
CHARACTERS
Appearance
White or almost white, crystalline powder.
Solubility
Freely soluble in water, practically insoluble in ethanol (96 per cent).♦
IDENTIFICATION
First identification: A, ♢ D.
Second identification: B, C, D.♢
A. Infrared absorption spectrophotometry (2.2.24).
Comparison: anhydrous lactose CRS.
B. Thin-layer chromatography (2.2.27).
Solvent mixture water R, methanol R (40:60 V/V).
Test solution: Dissolve 10 mg of the substance to be examined in the solvent mixture and dilute to 20 mL with the solvent mixture.
Reference solution: Dissolve 10 mg of anhydrous lactose CRS in the solvent mixture and dilute to 20 mL with the solvent mixture.
Plate: TLC silica gel plate R.
Mobile phase: water R, methanol R, glacial acetic acid R, methylene chloride R (10:15:25:50 V/V/V/V); measure the volumes accurately, as a slight excess of water produces cloudiness.
Application: 2 μL; thoroughly dry the points of application.
Development A: Over 3/4 of the plate.
Drying A: In a current of warm air.
Development B: Immediately, over 3/4 of the plate, after renewing the mobile phase.
Drying B: In a current of warm air.
Detection: Spray with a solution of 0.5 g of thymol R in a mixture of 5 mL of sulfuric acid R and 95 mL of ethanol (96 per cent) R; heat at 130 °C for 10 min.
Results: The principal spot in the chromatogram obtained with the test solution is similar in position, colour and size to the principal spot in the chromatogram obtained with the reference solution.
C. Dissolve 0.25 g in 5 mL of water R. Add 5 mL of ammonia R and heat in a water-bath at 80 °C for 10 min. A red colour develops.
D. Water (see Tests).♢
TESTS
Solution S
Dissolve 1.0 g in boiling water R, allow to cool and dilute to 10.0 mL with water R.
Appearance of solution
Solution S is clear (2.2.1) and not more intensely coloured than reference solution BY7 (2.2.2, Method II).
Acidity or alkalinity
Dissolve 6.0 g by heating in 25 mL of carbon dioxide-free water R, cool and add 0.3 mL of phenolphthalein solution R1. The solution is colourless. Not more than 0.4 mL of 0.1 M sodium hydroxide is required to change the colour of the indicator to pink or red.
Specific optical rotation (2.2.7)
+ 54.4 to + 55.9 (anhydrous substance).
Dissolve 10.0 g in 80 mL of water R with heating at 50 °C. Allow to cool and add 0.2 mL of dilute ammonia R1. Allow to stand for 30 min and dilute to 100.0 mL with water R.
Absorbance: proteins and light-absorbing impurities (2.2.25)
Test solution (a): Solution S.
Test solution (b): Dilute 1.0 mL of test solution (a) to 10.0 mL with water R.
Spectral range 400 nm for test solution (a) and 210-300 nm for test solution (b).
Results:
— at 400 nm: maximum 0.04 for test solution (a);
— from 210 nm to 220 nm: maximum 0.25 for test solution (b);
— from 270 nm to 300 nm: maximum 0.07 for test solution (b).
Water (2.5.12)
Maximum 1.0 per cent, determined on 1.00 g, using a mixture of 1 volume of formamide R and 2 volumes of methanol R as solvent.
Sulfated ash (2.4.14)
Maximum 0.1 per cent, determined on 1.0 g.
Microbial contamination
TAMC: acceptance criterion 10 CFU/g (2.6.12).
Absence of: Escherichia coli (2.6.13).
FUNCTIONALITY-RELATED CHARACTERISTICS
This section provides information on characteristics that are recognised as being relevant control parameters for one or more functions of the substance when used as an excipient (see chapter 5.15
Some of the characteristics described in the Functionality-related characteristics section may also be present in the mandatory part of the monograph since they also represent mandatory quality criteria. In such cases, a cross-reference to the tests described in the mandatory part is included in the Functionality-related characteristics section. Control of the characteristics can contribute to the quality of a medicinal product by improving the consistency of the manufacturing process and the performance of the medicinal product during use. Where control methods are cited, they are recognised as being suitable for the purpose, but other methods can also be used. Wherever results for a particular characteristic are reported, the control method must be indicated.
The following characteristics may be relevant for lactose used as filler/diluent in solid dosage forms (compressed and powder).
Particle-size distribution (2.9.31 or 2.9.38)
Bulk density of powders (2.9.34)
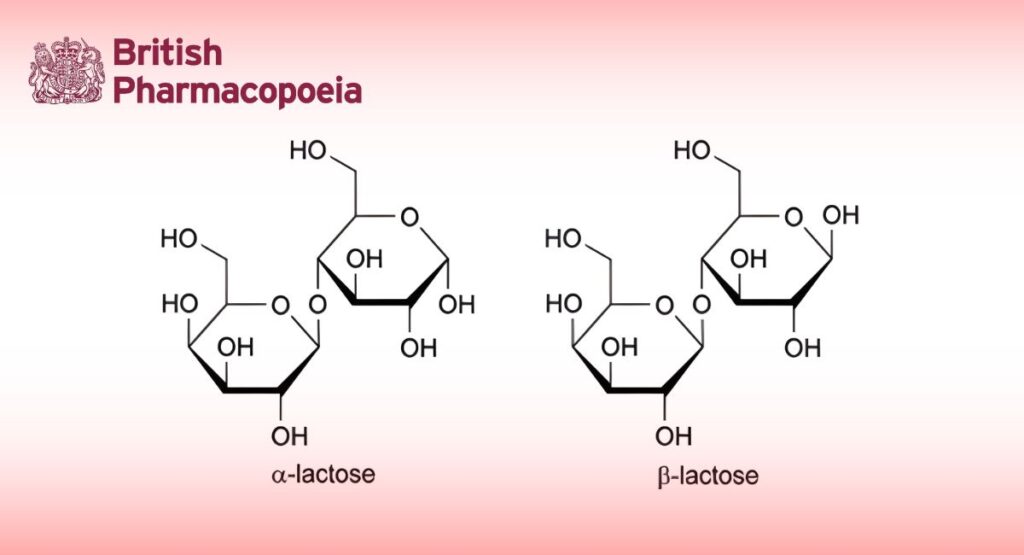
α-Lactose and β-lactose
Gas chromatography (2.2.28).
Silylation reagent dimethyl sulfoxide R, N-trimethylsilylimidazole R, pyridine R (19.5:22:58.5 V/V/V).
Test solution: Introduce 10 mg of the substance to be examined into a vial with a screw cap and add 4 mL of the silylation reagent. Sonicate for 20 min at room temperature, allow to cool and transfer 400 μL to an injection vial. Add 1 mL of pyridine R, close the vial and mix well.
Reference solution: Prepare a mixture of α-lactose monohydrate R and β-lactose R to obtain an anomeric ratio of about 1:1 based on the labelled anomeric contents of the α-lactose monohydrate and the β-lactose.
Introduce 10 mg of the mixture into a vial with a screw cap and add 4 mL of the silylation reagent. Sonicate for 20 min at room temperature, allow to cool, and transfer 400 μL to an injection vial. Add 1 mL of pyridine R, close the vial and mix well.
Precolumn:
— material: intermediate-polarity deactivated fused silica;
— size: l = 2 m, Ø = 0.53 mm.
Column:
— material: fused silica;
— size: l = 15 m, Ø = 0.25 mm;
— stationary phase: phenyl(5)methyl(95)polysiloxane R (film thickness 0.25 μm).
Carrier: gas helium for chromatography R.
Flow rate: 2.8 mL/min.
Temperature:
| Time
(min) |
Temperature
(°C) |
|
| Column | 0 – 1 | 80 |
| 1 – 3 | 80 → 150 | |
| 3 – 15.5 | 150 → 300 | |
| 15.5 – 17.5 | 300 | |
| Injection port | 275 or use cold on-column injection | |
| Detector | 325 |
Detection: Flame ionisation.
Injection: 0.5 μL, splitless or by cold on-column injection.
Relative retention: With reference to β-lactose (retention time = about 12 min): α-lactose = about 0.9.
System suitability: Reference solution:
— resolution: minimum 3.0 between the peaks due to α-lactose and β-lactose.
Calculate the percentage content of α-lactose using the following expression:
100 Sa/ (Sa + Sb)
Calculate the percentage content of β-lactose using the following expression:
100 Sb/ (Sa + Sb)
Sa = area of the peak due to α-lactose;
Sb = area of the peak due to β-lactose.
Loss on drying (2.2.32)
Determine on 1.000 g by drying in an oven at 80 °C for 2 h.
1 This monograph has undergone pharmacopoeial harmonisation. See chapter 5.8. Pharmacopoeial harmonisation.