Edition: BP 2025 (Ph. Eur. 11.6 update)
Action and use
Antiepileptic.
DEFINITION
Tablets containing Lacosamide (2992), for human use.
They comply with the monograph Tablets (0478) and the following additional requirements.
Content
95.0 per cent to 105.0 per cent of the content of lacosamide (C13H18N2O3) stated on the label.
IDENTIFICATION
A. Record the UV spectrum of the principal peak in the chromatograms obtained with the solutions used in the assay, with a diode array detector in the range of 210-400 nm.
Results The UV spectrum of the principal peak in the chromatogram obtained with the test solution is similar to the UV spectrum of the principal peak in the chromatogram obtained with reference solution (a).
B. Examine the chromatograms obtained in the assay.
Results The principal peak in the chromatogram obtained with the test solution is similar in retention time and size to the principal peak in the chromatogram obtained with reference solution (a).
TESTS
Related substances
Liquid chromatography (2.2.29).
Solvent mixture acetonitrile R, water R (13:87 V/V).
Test solution To 10 tablets add a suitable volume of the solvent mixture to obtain a concentration of lacosamide of 2- 4 mg/mL. Shake vigorously for 30 min, sonicate for 10 min and allow to stand for 30 min. Dilute a suitable volume of the supernatant with the solvent mixture to obtain a concentration of lacosamide of 1.0 mg/mL.
Reference solution (a) Dissolve 20.0 mg of lacosamide CRS in the solvent mixture and dilute to 20.0 mL with the solvent mixture.
Reference solution (b) Dilute 1.0 mL of the test solution to 100.0 mL with the solvent mixture. Dilute 2.0 mL of this solution to 10.0 mL with the solvent mixture.
Reference solution (c) Dissolve 2 mg of lacosamide impurity D CRS and 3 mg of lacosamide impurity F CRS in the solvent mixture and dilute to 100 mL with the solvent mixture. Dilute 1 mL of the solution to 10 mL with the solvent mixture.
Column:
— size: l = 0.15 m, Ø = 4.6 mm;
— stationary phase: end-capped extra-dense bonded octylsilyl silica gel for chromatography R (5 μm);
— temperature: 35 °C.
Mobile phase methanesulfonic acid R, acetonitrile R1, water for chromatography R (0.75:130:870 V/V/V).
Flow rate 2.0 mL/min.
Detection Spectrophotometer at 215 nm.
Injection 5 μL of the test solution and reference solutions (b) and (c).
Run time 2.5 times the retention time of lacosamide.
Identification of impurities Use the chromatogram obtained with reference solution (c) to identify the peaks due to impurities D and F.
Relative retention With reference to lacosamide (retention time = about 6 min): impurity D = about 0.4; impurity F = about 0.5.
System suitability Reference solution (c):
— resolution: minimum 1.5 between the peaks due to impurities D and F.
Calculation of percentage contents:
— for each impurity, use the concentration of lacosamide in reference solution (b).
Limits:
— unspecified impurities: for each impurity, maximum 0.2 per cent;
— total: maximum 1.0 per cent;
— reporting threshold: 0.1 per cent.
Dissolution1 (2.9.3, Apparatus 2).
Dissolution medium 10.3 g/L solution of hydrochloric acid R. Use 900 mL of the medium.
Rotation speed 50 r/min.
Time 30 min.
Analysis Liquid chromatography (2.2.29).
Test solutions Samples withdrawn from the dissolution vessel and filtered.
Reference solution Using sonication, dissolve a suitable quantity of lacosamide CRS in a suitable volume of the dissolution medium to obtain a concentration of lacosamide corresponding to the theoretical concentration of lacosamide in the test solution, based on the labelled content of the tablets.
Column:
— size: l = 0.05 m, Ø = 4.6 mm;
— stationary phase: end-capped octadecylsilyl silica gel for chromatography R (3 μm);
— temperature: 35 °C.
Mobile phase trifluoroacetic acid R, acetonitrile R1, water for chromatography R (1:300:700 V/V/V).
Flow rate 1.0 mL/min.
Detection Spectrophotometer at 215 nm.
Injection 2 μL.
Run time 2.5 min.
System suitability Reference solution:
— repeatability: maximum relative standard deviation of 1.5 per cent determined on 6 injections.
Calculate the amount of dissolved lacosamide (C13H18N2O3), expressed as a percentage of the content stated on the label,
taking into account the assigned content of lacosamide CRS.
Acceptance criterion:
— Q = 80 per cent after 30 min.
ASSAY
Liquid chromatography (2.2.29) as described in the test for related substances with the following modifications.
Injection Test solution and reference solution (a).
System suitability Reference solution (a):
— repeatability: maximum relative standard deviation of 1.5 per cent determined on 6 injections.
Calculate the percentage content of lacosamide (C13H18N2O3) taking into account the assigned content of lacosamide CRS.
IMPURITIES
Other detectable impurities (the following substances would, if present at a sufficient level, be detected by one or other of the tests in the monograph): B, C, D, E, F, J, K.
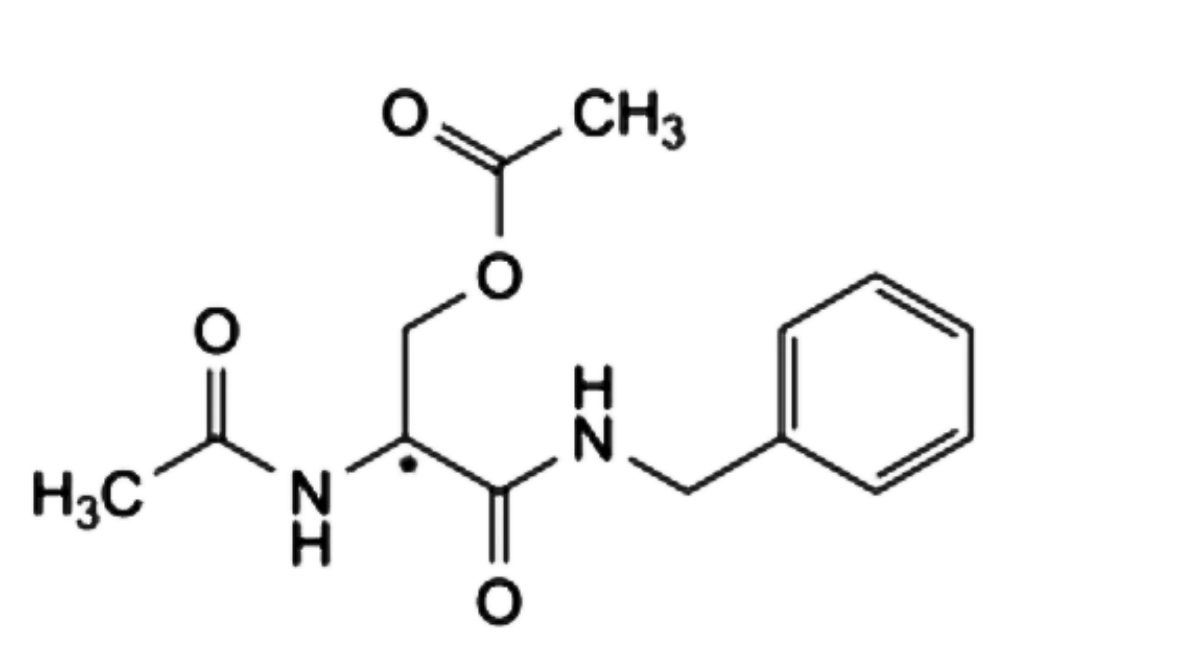
B. (2Ξ)-2-acetamido-3-(benzylamino)-3-oxopropyl acetate,
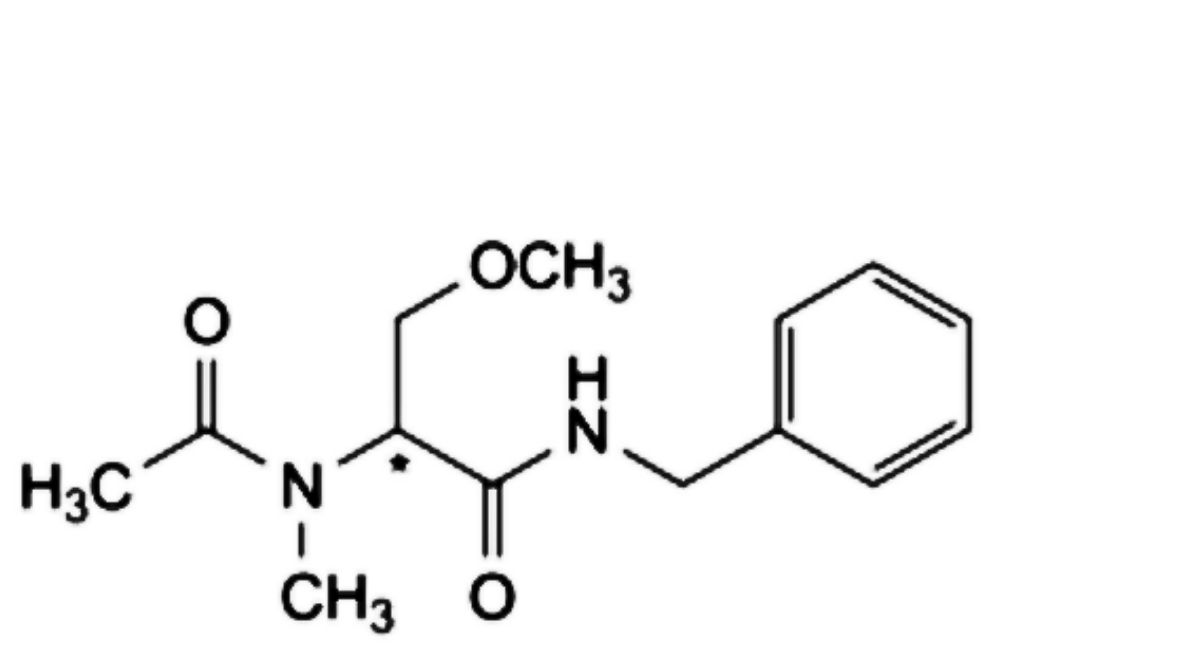
C. (2Ξ)-N-benzyl-3-methoxy-2-(N-methylacetamido)propanamide,
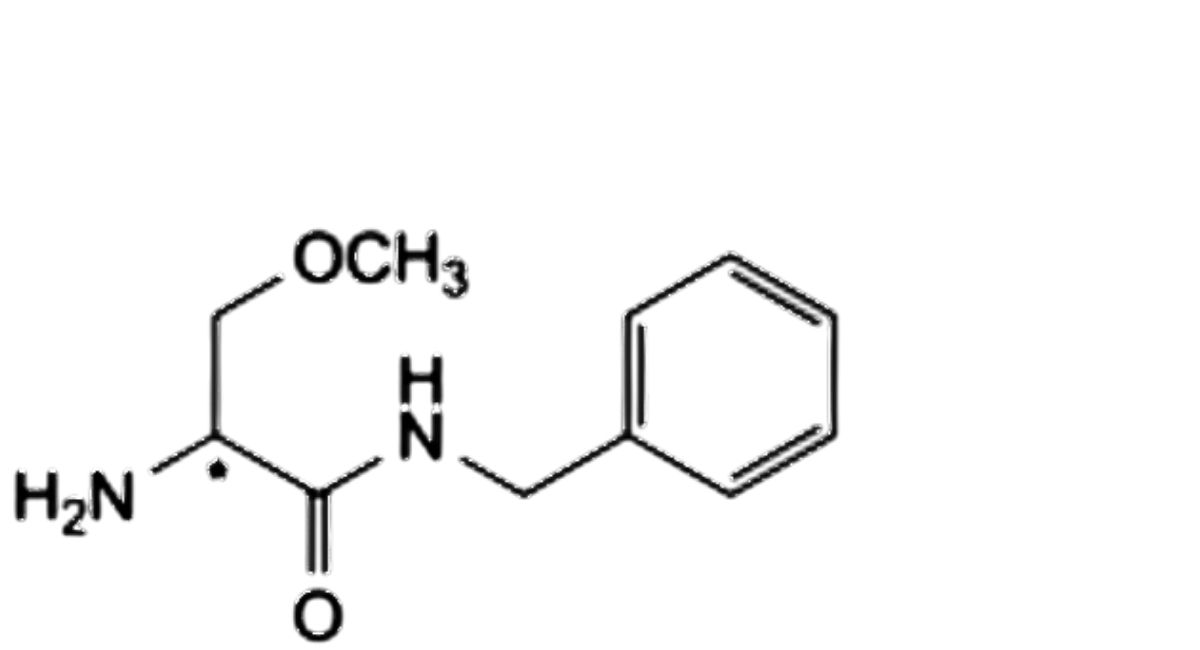
D. (2Ξ)-2-amino-N-benzyl-3-methoxypropanamide,
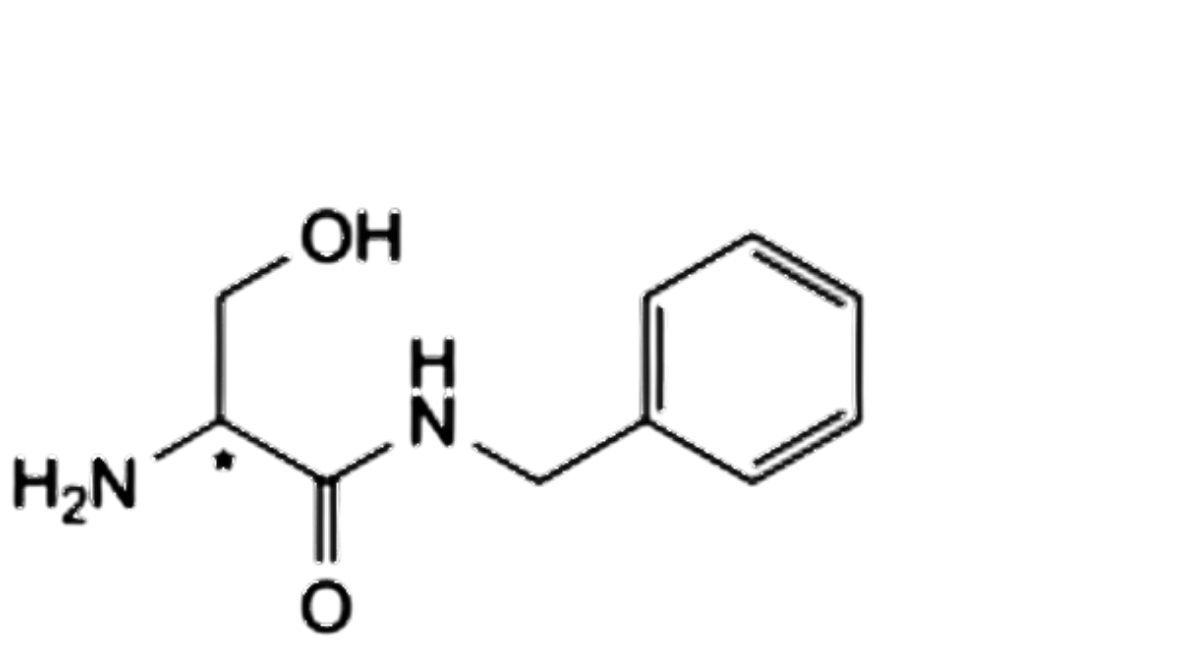
E. (2Ξ)-2-amino-N-benzyl-3-hydroxypropanamide,
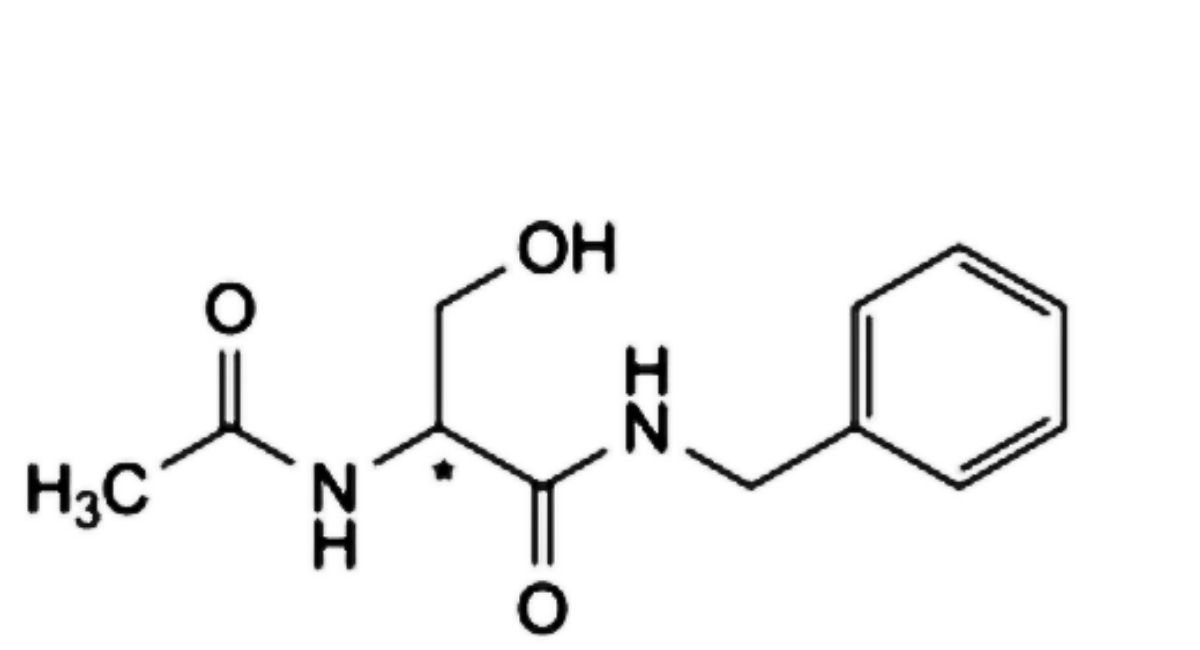
F. (2Ξ)-2-acetamido-N-benzyl-3-hydroxypropanamide,
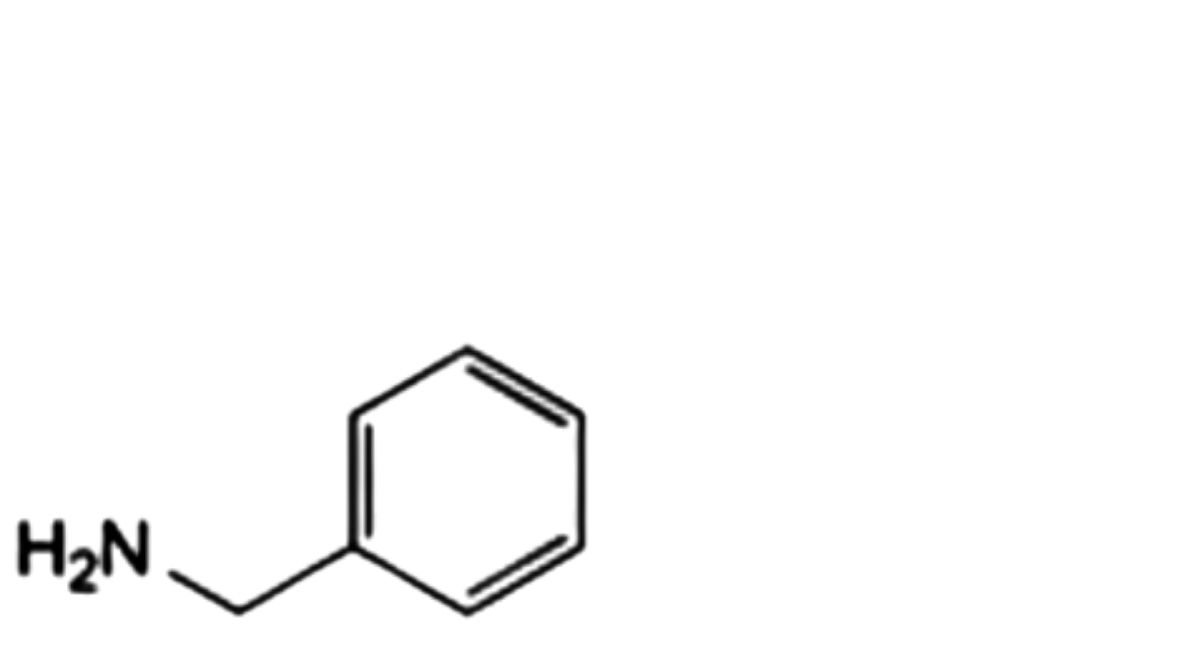
J. phenylmethanamine,
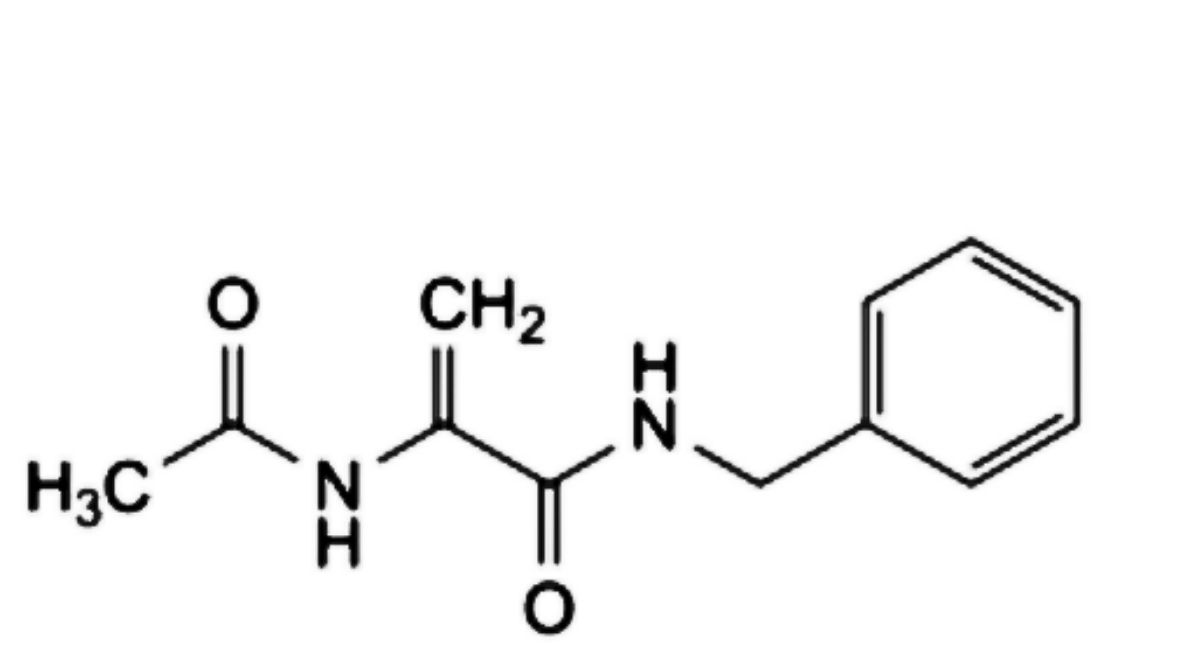
K. 2-acetamido-N-benzylprop-2-enamide.






