Edition: BP 2025 (Ph. Eur. 11.6 update)
Action and use
Antiepileptic.
DEFINITION
Oral solution of Lacosamide (2992), for human use.
It complies with the monograph Liquid preparations for oral use (0672) and the following additional requirements.
Content
95.0 per cent to 105.0 per cent of the content of lacosamide (C13H18N2O3) stated on the label.
IDENTIFICATION
A. Record the UV spectrum of the principal peak in the chromatograms obtained with the solutions used in the assay, with a diode array detector in the range of 210-400 nm.
Results The UV spectrum of the principal peak in the chromatogram obtained with the test solution is similar to the UV spectrum of the principal peak in the chromatogram obtained with reference solution (a).
B. Examine the chromatograms obtained in the assay.
Results The principal peak in the chromatogram obtained with the test solution is similar in retention time and size to the principal peak in the chromatogram obtained with reference solution (a).
TESTS
Related substances
Liquid chromatography (2.2.29).
Solvent mixture acetonitrile R, water R (13:87 V/V).
Test solution Dilute a suitable volume of the preparation to be examined with the solvent mixture to obtain a concentration of lacosamide of 1.0 mg/mL.
Reference solution (a) Dissolve 20.0 mg of lacosamide CRS in the solvent mixture and dilute to 20.0 mL with the solvent mixture.
Reference solution (b) Dilute 1.0 mL of the test solution to 100.0 mL with the solvent mixture. Dilute 2.0 mL of this solution to 10.0 mL with the solvent mixture.
Reference solution (c) Dissolve 2 mg of lacosamide impurity D CRS and 3 mg of lacosamide impurity F CRS in the solvent mixture and dilute to 100 mL with the solvent mixture. Dilute 1 mL of the solution to 10 mL with the solvent mixture.
Column:
— size: l = 0.25 m, Ø = 4.6 mm;
— stationary phase: end-capped octadecylsilyl silica gel for chromatography compatible with 100 per cent aqueous mobile phases R (5 μm);
— temperature: 30 °C.
Mobile phase:
— mobile phase A: trifluoroacetic acid R, acetonitrile R1, water for chromatography R (0.56:100:900 V/V/V);
— mobile phase B: trifluoroacetic acid R, acetonitrile R1 (0.5:1000 V/V);
| Time
(min) |
Mobile phase A
(per cent V/V) |
Mobile phase B
(per cent V/V) |
| 0 – 31
31 – 33 |
100
30 |
0
70 |
Flow rate 1.5 mL/min.
Detection Spectrophotometer at 215 nm.
Injection 5 μL of the test solution and reference solutions (b) and (c).
Identification of impurities Use the chromatogram obtained with reference solution (c) to identify the peaks due to impurities D and F; impurities D and F may be inverted in the elution order, but the peak area of each impurity is different, so a clear identification of the impurities is possible.
Relative retention With reference to lacosamide (retention time = about 27 min): impurity D = about 0.4; impurity F = about 0.5 (D and F may be inverted).
System suitability Reference solution (c):
— resolution: minimum 3.0 between the peaks due to impurities D and F.
Calculation of percentage contents:
— for each impurity, use the concentration of lacosamide in reference solution (b).
Limits:
— impurity D: maximum 0.6 per cent;
— unspecified impurities: for each impurity, maximum 0.2 per cent;
— total: maximum 2.0 per cent;
— reporting threshold: 0.1 per cent.
ASSAY
Liquid chromatography (2.2.29) as described in the test for related substances with the following modifications.
Mobile phase:
— mobile phase A: 0.05 per cent V/V solution of trifluoroacetic acid R;
— mobile phase B: trifluoroacetic acid R, acetonitrile R1 (0.5:1000 V/V);
| Time
(min) |
Mobile phase A
(per cent V/V) |
Mobile phase B
(per cent V/V) |
| 0 – 9
9 – 12.5 |
75
45 |
25
55 |
Injection 4 μL of the test solution and reference solution (a).
System suitability Reference solution (a):
— repeatability: maximum relative standard deviation of 1.5 per cent determined on 6 injections.
Calculate the percentage content of lacosamide (C13H18N2O3) taking into account the assigned content of lacosamide CRS.
IMPURITIES
Specified impurities D.
Other detectable impurities (the following substances would, if present at a sufficient level, be detected by one or other of the tests in the monograph): E, F, G, J, K.
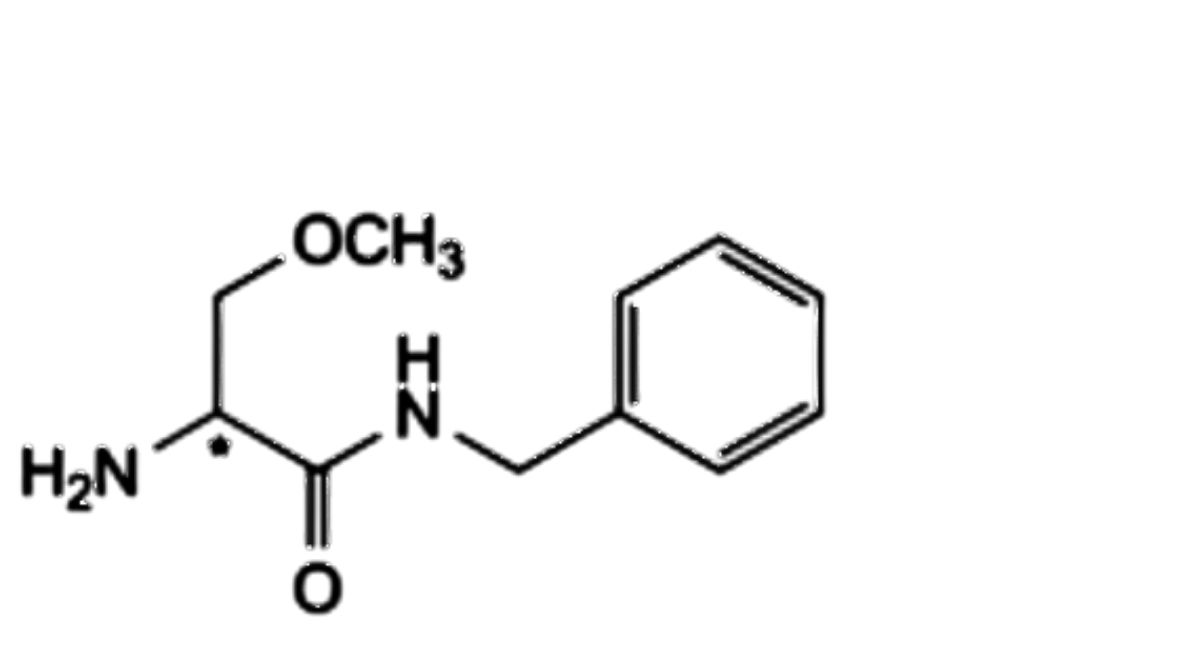
D. (2Ξ)-2-amino-N-benzyl-3-methoxypropanamide,
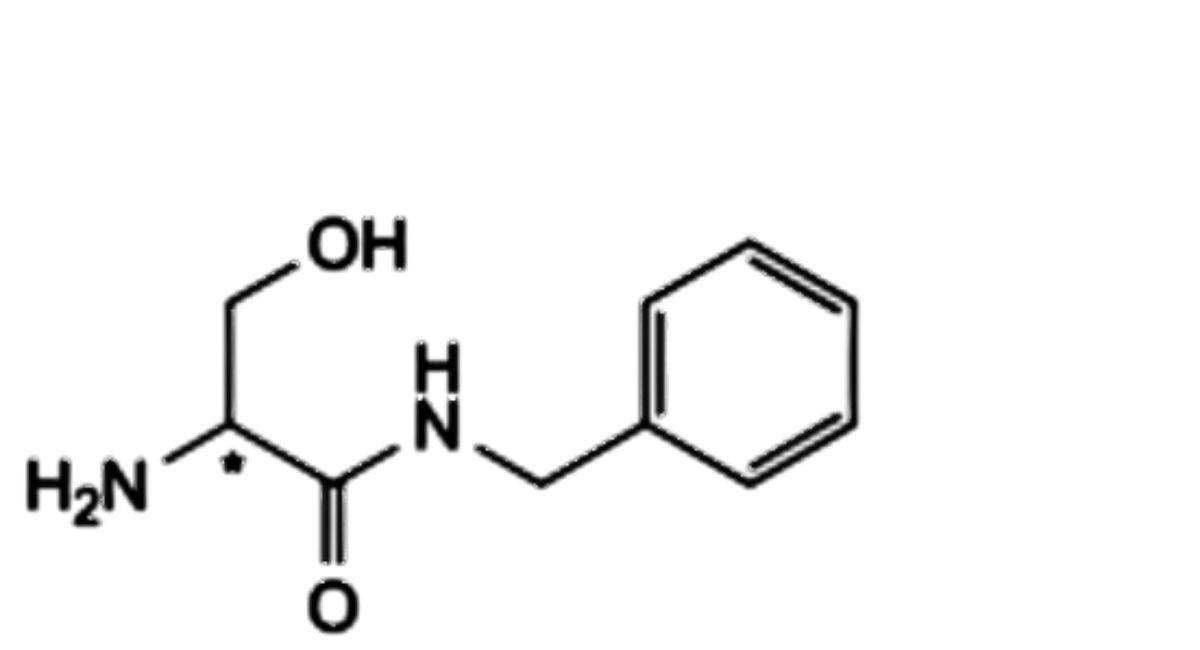
E. (2Ξ)-2-amino-N-benzyl-3-hydroxypropanamide,
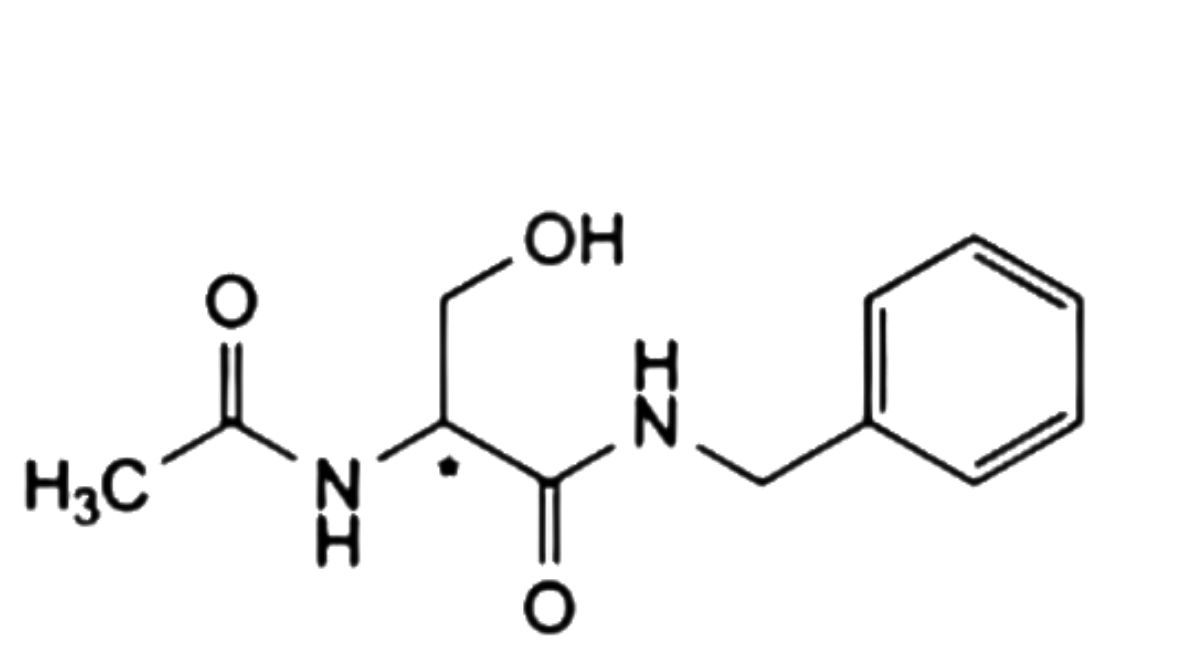
F. (2Ξ)-2-acetamido-N-benzyl-3-hydroxypropanamide,
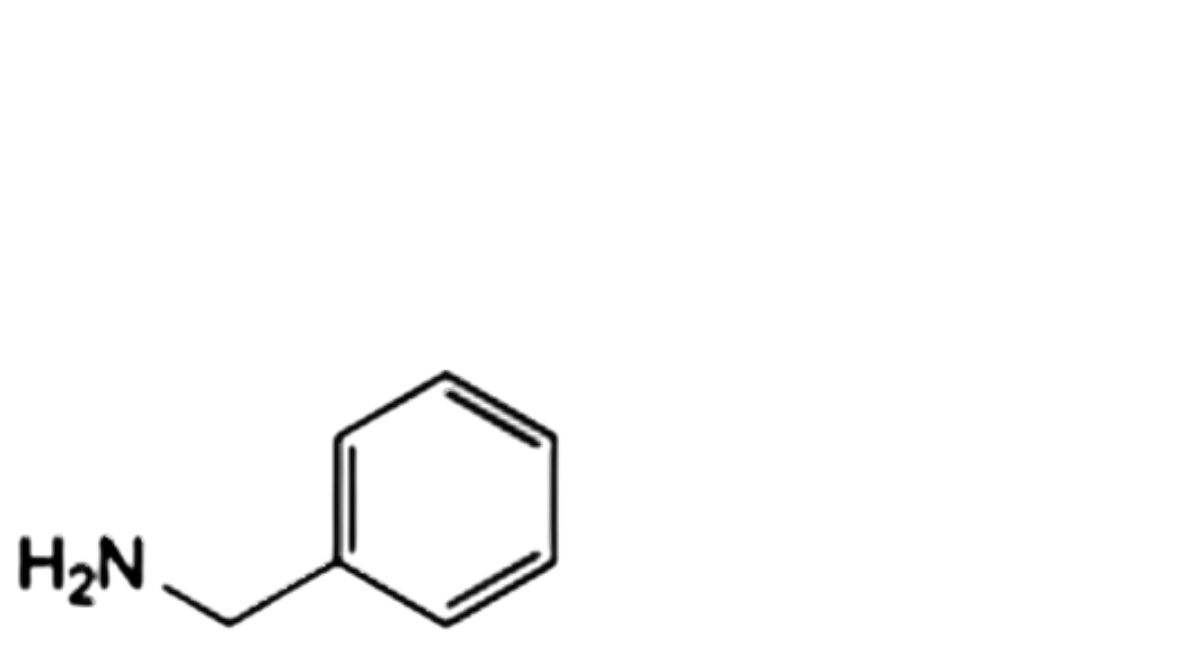
G. N-benzylacetamide,
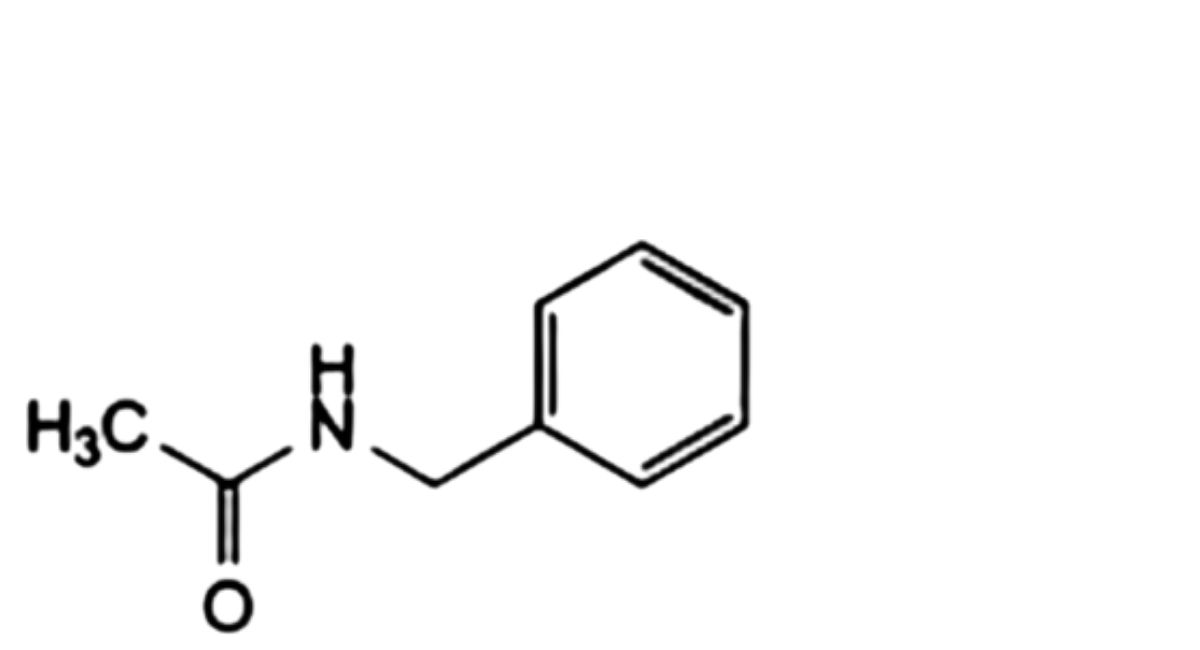
J. phenylmethanamine,
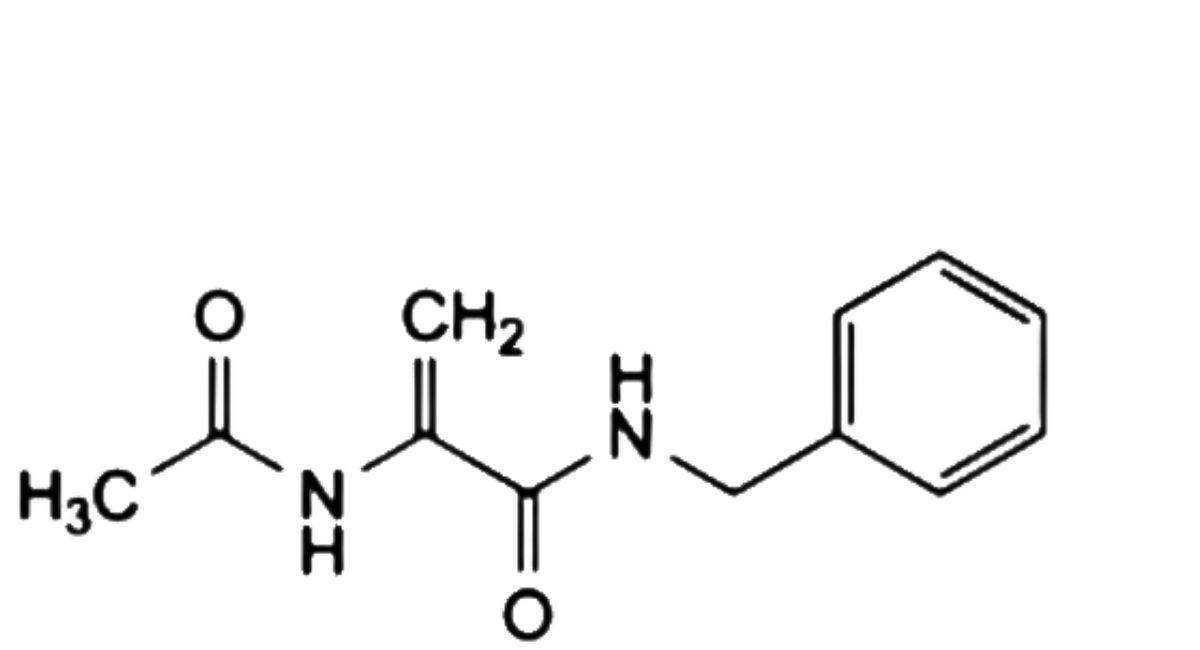
K. 2-acetamido-N-benzylprop-2-enamide.






