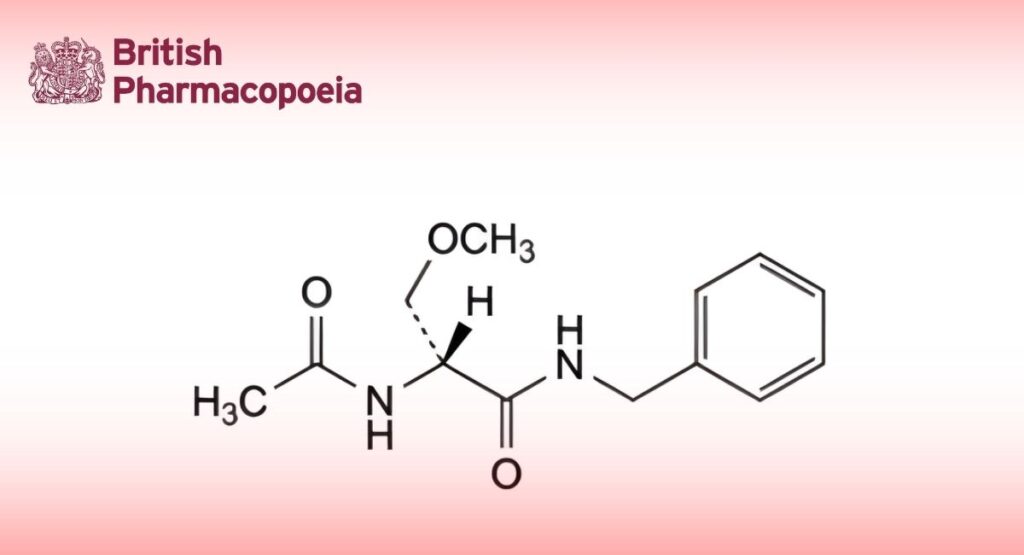
(Ph. Eur. monograph 2992)
C13H18N2O3 250.3 175481-36-4
Action and use
Antiepileptic.
Preparations
Lacosamide Infusion
Lacosamide Oral Solution
Lacosamide Tablets
DEFINITION
(2R)-2-Acetamido-N-benzyl-3-methoxypropanamide.
Content
98.0 per cent to 102.0 per cent (anhydrous substance).
CHARACTERS
Appearance
White or almost white or light yellow powder.
Solubility
Sparingly soluble in water, freely soluble in methanol, practically insoluble in heptane.
It shows polymorphism (5.9).
IDENTIFICATION
Carry out either tests A, B or tests B, C.
A. Specific optical rotation (2.2.7): + 14 to + 18 (anhydrous substance), measured at 25 °C.
Dissolve 0.100 g in methanol R and dilute to 10.0 mL with the same solvent.
B. Infrared absorption spectrophotometry (2.2.24).
Comparison: lacosamide CRS.
If the spectra obtained in the solid state show differences, dissolve the substance to be examined and the reference substance separately in methanol R, evaporate to dryness and record new spectra using the residues.
C. Enantiomeric purity (see Tests).
TESTS
Appearance of solution
The solution is clear (2.2.1) and colourless (2.2.2, Method II).
Dissolve 0.500 g in water R and dilute to 50.0 mL with the same solvent.
Enantiomeric purity
Liquid chromatography (2.2.29): use the normalisation procedure.
Test solution: Dissolve 50.0 mg of the substance to be examined in the mobile phase and dilute to 50.0 mL with the mobile phase.
Reference solution (a): Dissolve 1 mg of lacosamide impurity A CRS in the mobile phase and dilute to 10.0 mL with the mobile phase.
Reference solution (b): Dissolve 20 mg of the substance to be examined in the mobile phase, add 1.0 mL of reference solution (a) and dilute to 20.0 mL with the mobile phase.
Reference solution (c): Dilute 1.0 mL of the test solution to 100.0 mL with the mobile phase. Dilute 1.0 mL of this solution to 20.0 mL with the mobile phase.
Column:
— size: l = 0.25 m, Ø = 4.6 mm;
— stationary phase: amylose derivative of silica gel for chiral separation R (10 μm).
Mobile phase: water for chromatography R, 2-propanol R, heptane R (3:100:900 V/V/V).
Flow rate: 1.0 mL/min.
Detection: Spectrophotometer at 215 nm.
Injection: 20 μL of the test solution and reference solutions (b) and (c).
Run time: 1.7 times the retention time of lacosamide.
Identification of impurities: Use the chromatogram obtained with reference solution (b) to identify the peak due to impurity A.
Relative retention: With reference to lacosamide (retention time = about 25 min): impurity A = about 0.8.
System suitability: Reference solution (b):
— resolution: minimum 3.0 between the peaks due to impurity A and lacosamide.
Limit:
— impurity A: maximum 0.15 per cent;
— reporting threshold: 0.05 per cent (reference solution (c)).
Related substances
Liquid chromatography (2.2.29).
Test solution: Dissolve 50.0 mg of the substance to be examined in 1 mL of methanol R and dilute to 10.0 mL with water R.
Reference solution (a): Dissolve 50.0 mg of lacosamide CRS in 1 mL of methanol R and dilute to 10.0 mL with water R.
Reference solution (b): Dilute 1.0 mL of the test solution to 100.0 mL with a mixture of 10 volumes of methanol R and 90 volumes of water R. Dilute 1.0 mL of this solution to 10.0 mL with the same mixture of solvents.
Reference solution (c): Dissolve 5 mg of lacosamide for system suitability CRS (containing impurities B, C, G and I) in 0.1 mL of methanol R and dilute to 1.0 mL with water R.
Reference solution (d): Dissolve 1 mg of lacosamide impurity F CRS in 2 mL of methanol R and dilute to 10.0 mL with water R. Dilute 1.0 mL of the solution to 10.0 mL with water R.
Column:
— size: l = 0.15 m, Ø = 4.6 mm;
— stationary phase: end-capped extra-dense bonded octylsilyl silica gel for chromatography R (3.5 μm).
Mobile phase:
— mobile phase A: 0.1 per cent V/V solution of trifluoroacetic acid R;
— mobile phase B: trifluoroacetic acid R, acetonitrile R, methanol R (0.3:500:500 V/V/V);
| Time
(min) |
Mobile phase A
(per cent V/V) |
Mobile phase B
(per cent V/V) |
| 0 – 2 | 89 | 11 |
| 2 – 14.2 | 89 → 69 | 11 → 31 |
| 14.2 – 19.5 | 69 → 23 | 31 → 77 |
| 19.5 – 20 | 23 → 0 | 77 → 100 |
| 20 – 21 | 0 | 100 |
Flow rate: 1.2 mL/min.
Detection: Spectrophotometer at 258 nm.
Injection: 20 μL of the test solution and reference solutions (b), (c) and (d).
Identification of impurities: Use the chromatogram supplied with lacosamide for system suitability CRS and the chromatogram obtained with reference solution (c) to identify the peaks due to impurities B, C, G and I; use the chromatogram obtained with reference solution (d) to identify the peak due to impurity F.
Relative retention: With reference to lacosamide (retention time = about 12 min): impurity F = about 0.7; impurity G = about 0.9; impurity B = about 1.2; impurity C = about 1.3; impurity I = about 1.6.
System suitability: Reference solution (c):
— resolution: minimum 2.0 between the peaks due to impurity G and lacosamide; minimum 4.5 between the peaks due to lacosamide and impurity B.
Calculation of percentage contents:
— correction factors: multiply the peak areas of the following impurities by the corresponding correction factor: impurity G = 0.7; impurity I = 0.7;
— for each impurity, use the concentration of lacosamide in reference solution (b).
Limits:
— impurities B, C, F, G, I: for each impurity, maximum 0.15 per cent;
— unspecified impurities: for each impurity, maximum 0.10 per cent;
— total: maximum 0.5 per cent;
— reporting threshold: 0.05 per cent.
Water (2.5.32)
Maximum 0.2 per cent, determined on 0.150 g by direct sample introduction.
Sulfated ash (2.4.14)
Maximum 0.1 per cent, determined on 1.0 g.
ASSAY
Liquid chromatography (2.2.29) as described in the test for related substances with the following modifications.
Injection: Test solution and reference solution (a).
System suitability Reference solution (a):
— symmetry factor: maximum 2.4 for the peak due to lacosamide.
Calculate the percentage content of C13H18N2O3 taking into account the assigned content of lacosamide CRS.
IMPURITIES
Specified impurities A, B, C, F, G, I.
Other detectable impurities (the following substances would, if present at a sufficient level, be detected by one or other of the tests in the monograph. They are limited by the general acceptance criterion for other/unspecified impurities and/or by the general monograph Substances for pharmaceutical use (2034). It is therefore not necessary to identify these impurities for demonstration of compliance. See also 5.10. Control of impurities in substances for pharmaceutical use) D, E, H, J, K.
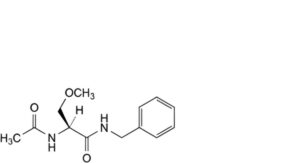
A. (2S)-2-acetamido-N-benzyl-3-methoxypropanamide,
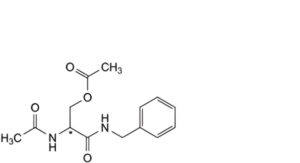
B. (2Ξ)-2-acetamido-3-(benzylamino)-3-oxopropyl acetate,
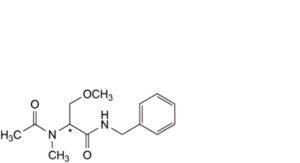
C. (2Ξ)-N-benzyl-3-methoxy-2-(N-methylacetamido)propanamide,
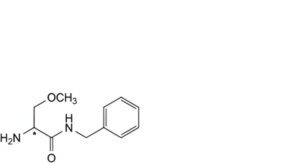
D. (2Ξ)-2-amino-N-benzyl-3-methoxypropanamide,
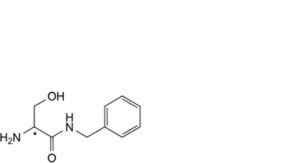
E. (2Ξ)-2-amino-N-benzyl-3-hydroxypropanamide,
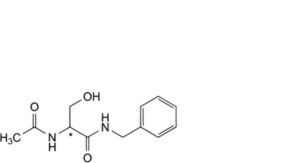
F. (2Ξ)-2-acetamido-N-benzyl-3-hydroxypropanamide,
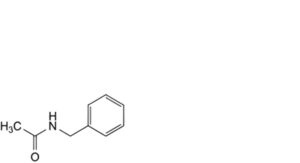
G. N-benzylacetamide,
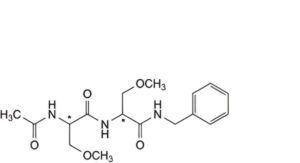
H. (2Ξ)-2-acetamido-N-[(2Ξ)-1-(benzylamino)-3-methoxy-1-oxopropan-2-yl]-3-methoxypropanamide,
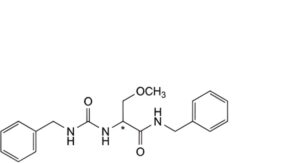
I. (2Ξ)-N-benzyl-2-[(benzylcarbamoyl)amino]-3-methoxypropanamide,
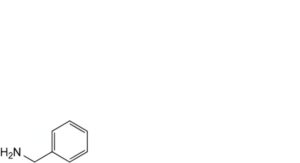
J. phenylmethanamine,
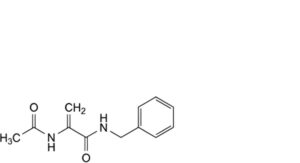
K. 2-acetamido-N-benzylprop-2-enamide.