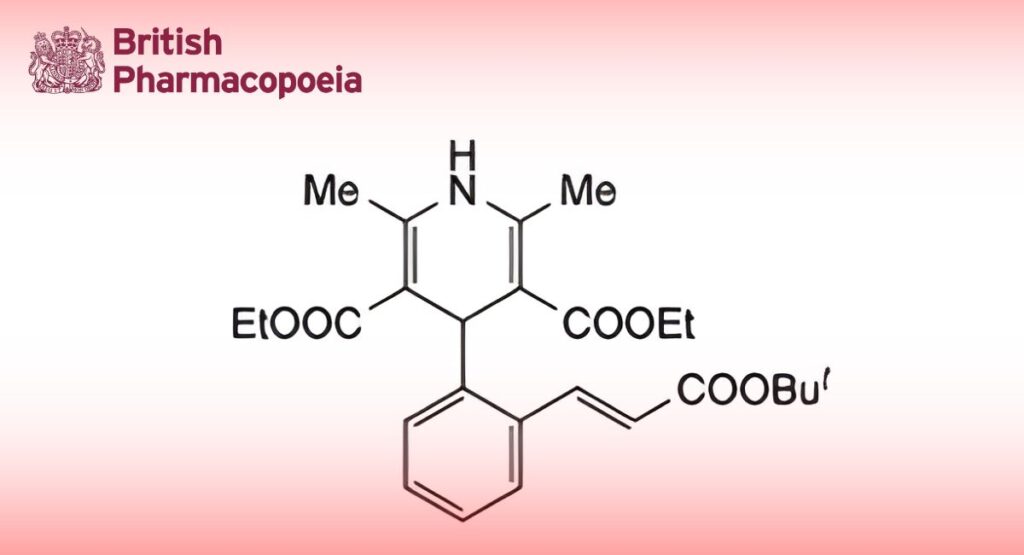
C26H33NO6 455.6 103890-78-4
Action and use
Calcium channel blocker.
Preparation
Lacidipine Tablets
DEFINITION
Lacidipine is diethyl (E)-4-{2-[(tert-butoxycarbonyl)vinyl]phenyl}-1,4-dihydro-2,6-dimethylpyridine-3,5- dicarboxylate. It contains not less than 97.5% and not more than 102.0% of C26H33NO6, calculated with reference to the anhydrous, propan-2-ol–free substance.
CHARACTERISTICS
A white to pale yellow crystalline powder. It melts at about 178°.
Practically insoluble in water; freely soluble in acetone; sparingly soluble in absolute ethanol.
Carry out all of the following procedures protected from light and prepare solutions immediately before use.
IDENTIFICATION
A. The infrared absorption spectrum, Appendix II A, is concordant with the reference spectrum of Lacidipine (RS 407).
B. In the Assay, the principal peak in the chromatogram obtained with solution (1) has the same retention time as the principal peak in the chromatogram obtained with solution (2).
TESTS
Propan-2-ol
Carry out the method for gas chromatography, Appendix III B. Prepare a 0.002% v/v solution of 1,4-dioxan (internal standard) in dimethylacetamide (solution A).
(1) 0.002% v/v solution of propan-2-ol in solution A.
(2) 2% w/v of the substance being examined in solution A.
CHROMATOGRAPHIC CONDITIONS
(a) Use a glass column (60 m × 0.32 mm) bonded with a film (5 μm) of polymethylsiloxane (CP-Sil 5CB is suitable).
(b) Use helium as the carrier gas at 1.7 mL per minute.
(c) Use a temperature gradient as described below.
(d) Use an injection temperature of 170°.
(e) Use a detector temperature of 250°.
(f) Inject 1 μL of each solution.
SYSTEM SUITABILITY
The test is not valid unless the chromatogram obtained with solution (1) shows two clearly separated peaks.
The retention time for propan-2-ol is about 6.2 minutes and that for dioxan is about 15 minutes.
LIMITS
In the chromatogram obtained with solution (2): the percentage content of propan-2-ol is not more than 0.5% w/w.
Related substances
Carry out the method for liquid chromatography, Appendix III D, using the following solutions.
(1) Dilute 1 volume of a 0.1% w/v solution of the substance being examined in absolute ethanol to 5 volumes with the mobile phase.
(2) Dilute 1 volume of solution (1) to 500 volumes with the mobile phase.
(3) Dilute 1 volume of a 0.1% w/v solution of lacidipine impurity standard BPCRS in absolute ethanol to 5 volumes with the mobile phase.
CHROMATOGRAPHIC CONDITIONS
(a) Use a stainless steel column (25 cm x 4.6 mm) packed with cyanosilyl silica gel for chromatography (5 μm) (Spherisorb CN is suitable).
(b) Use isocratic elution using the mobile phase described below.
(c) Use a flow rate of 2 mL per minute.
(d) Use an ambient column temperature.
(e) Use a detection wavelength of 240 nm.
(f) Inject 20 μL of each solution.
(g) If necessary adjust the composition of the mobile phase so that, in the chromatogram obtained with solution (3), the retention time of the peak due to lacidipine is about 10 minutes.
(h) For solution (1), allow the chromatography to proceed for 2 times the retention time of the principal peak.
MOBILE PHASE
3 volumes of absolute ethanol and 97 volumes of n-hexane.
SYSTEM SUITABILITY
The test is not valid unless the chromatogram obtained with solution (3) closely resembles the chromatogram supplied with lacidipine impurity standard BPCRS.
LIMITS
In the chromatogram obtained with solution (1):
the area of any peak due to lacidipine impurity B is not greater than 0.5 times the area of the principal peak in the chromatogram obtained with solution (2) (0.2%, taking into account the correction factor of 0.5);
the area of any other secondary peak is not greater than the area of the principal peak in the chromatogram obtained with solution (2) (0.2%);
the total nominal content of impurities is not greater than 0.5%.
Water
Not more than 0.2% w/w, Appendix IX C. Use 0.5 g.
ASSAY
Carry out the method for liquid chromatography, Appendix III D, using the following solutions.
(1) Dilute 5 volumes of a 0.1% w/v solution of the substance being examined in absolute ethanol to 100 volumes with the mobile phase.
(2) Dilute 5 volumes of a 0.1% w/v solution of lacidipine BPCRS in absolute ethanol to 100 volumes with the mobile phase.
(3) Dilute 1 volume of a 0.1% w/v solution of lacidipine impurity standard BPCRS in absolute ethanol to 5 volumes with the mobile phase.
CHROMATOGRAPHIC CONDITIONS
The chromatographic procedure described under Related substances may be used.
SYSTEM SUITABILITY
The test is not valid unless the chromatogram obtained with solution (3) closely resembles the corresponding chromatogram supplied with lacidipine impurity standard BPCRS.
DETERMINATION OF CONTENT
Calculate the content of C26H33NO6 from the chromatograms obtained and using the declared content of C26H33NO6 in lacidipine BPCRS.
IMPURITIES
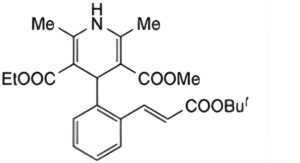
A. Ethyl methyl (E)-4-{2-[2-(tert-butoxycarbonyl)vinyl]phenyl}-1,4-dihydro-2,6-dimethylpyridine-3,5- dicarboxylate,
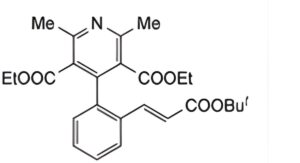
B. Diethyl (E)-4-{2-[2-(tert-butoxycarbonyl)vinyl]phenyl}-2,6-dimethylpyridine-3,5-dicarboxylate,
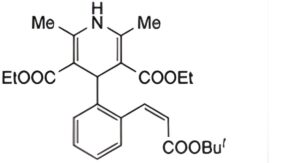
C. Diethyl (Z)-4-{2-[2-(tert-butoxycarbonyl)vinyl]phenyl}-1,4-dihydro-2,6-dimethylpyridine-3,5-dicarboxylate.