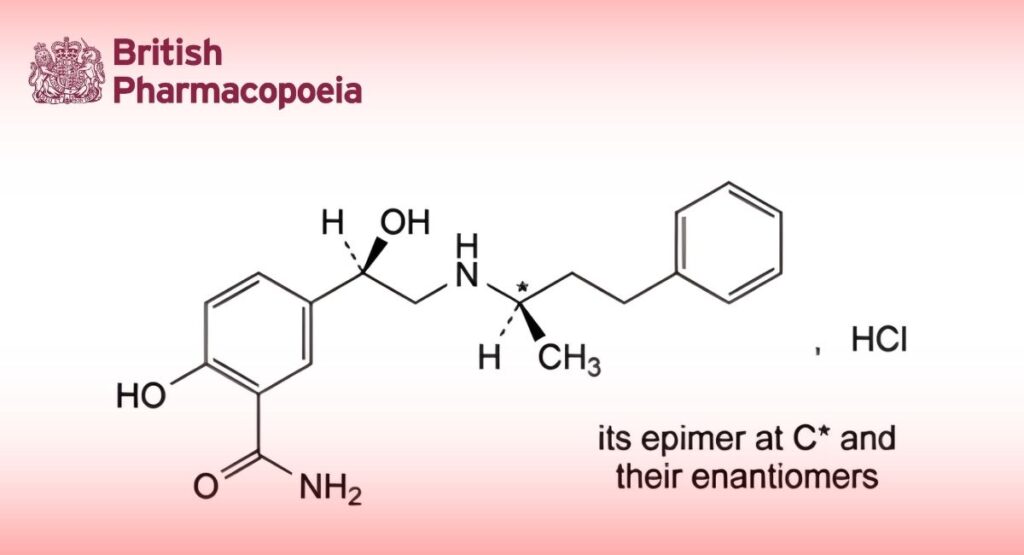
(Ph. Eur. monograph 0923)
C19H25ClN2O3 364.9 32780-64-6
Action and use
Alpha-and beta-adrenoceptor antagonist.
Preparations
Labetalol Injection
Labetalol Tablets
DEFINITION
Mixture of 4 stereoisomers of 2-hydroxy-5-[(1Ξ)-1-hydroxy-2-[[(2Ξ)-4-phenylbutan-2-yl]amino]ethyl]benzamide hydrochloride.
Content
98.0 per cent to 102.0 per cent (dried substance).
CHARACTERS
Appearance
White or almost white powder.
Solubility
Sparingly soluble in water and in ethanol (96 per cent), practically insoluble in methylene chloride.
IDENTIFICATION
First identification: A, C, E.
Second identification: A, B, D, E.
A. Optical rotation (2.2.7): -0.05° to + 0.05°, determined on solution S (see Tests).
B. Ultraviolet and visible absorption spectrophotometry (2.2.25).
Test solution: Dissolve 25.0 mg in a 10.3 g/L solution of hydrochloric acid R and dilute to 250.0 mL with the same solution.
Spectral range: 230-350 nm.
Absorption maximum: 302 nm.
Specific absorbance at the absorption maximum 83 to 88.
C. Infrared absorption spectrophotometry (2.2.24).
Comparison: labetalol hydrochloride CRS.
D. Thin-layer chromatography (2.2.27).
Test solution: Dissolve 10 mg of the substance to be examined in 1 mL of ethanol (96 per cent) R.
Reference solution (a): Dissolve 10 mg of labetalol hydrochloride CRS in 1 mL of ethanol (96 per cent) R.
Reference solution (b): Dissolve 10 mg of labetalol hydrochloride CRS and 10 mg of propranolol hydrochloride CRS in ethanol (96 per cent) R and dilute to 5 mL with the same solvent.
Plate: TLC octadecylsilyl silica gel F254 plate R.
Mobile phase: perchloric acid R, water R, methanol R (0.5:50:80 V/V/V).
Application: 2 μL.
Development: Place the plate in a chromatographic tank immediately after the addition of the mobile phase, close the tank and develop over 3/4 of the plate.
Drying: In air.
Detection: Examine in ultraviolet light at 254 nm.
System suitability: Reference solution (b):
— the chromatogram shows 2 clearly separated spots.
Results: The principal spot in the chromatogram obtained with the test solution is similar in position and size to the principal spot in the chromatogram obtained with reference solution (a).
E. It gives reaction (a) of chlorides (2.3.1).
TESTS
Solution S
Dissolve 0.50 g in carbon dioxide-free water R and dilute to 50 mL with the same solvent. Solution S must be freshly prepared.
Appearance of solution
Solution S is clear (2.2.1) and not more intensely coloured than intensity 6 of the range of reference solutions of the most appropriate colour (2.2.2, Method II).
pH (2.2.3)4.0 to 5.0 for solution S.
Diastereoisomer ratio
Gas chromatography (2.2.28).
Test solution: Dissolve 2.0 mg of the substance to be examined in 1.0 mL of a 12.0 g/L solution of butylboronic acid R in anhydrous pyridine R and allow to stand for 20 min.
Column:
— material: glass;
— size: l = 1.5 m, Ø = 4 mm;
— stationary phase: silanised diatomaceous earth for gas chromatography R (125-150 μm) impregnated with 3 per cent m/m of phenyl(50)methyl(50)polysiloxane R.
Carrier: gas nitrogen for chromatography R.
Flow rate: 40 mL/min.
Temperature:
— column, injection port and detector: 300 °C.
Detection: Flame ionisation.
Injection: 2 μL.
System suitability:
— the height of the trough separating the 2 peaks due to the pairs of diastereoisomers is less than 5 per cent of the full scale of the recorder.
Limit:
— each pair of diastereoisomers: for the area of each peak, 45 per cent to 55 per cent of the total area of the 2 peaks.
Related substances
Liquid chromatography (2.2.29).
Test solution (a): Dissolve 25.0 mg of the substance to be examined in mobile phase A and dilute to 10.0 mL with mobile phase A.
Test solution (b): Dilute 1.0 mL of test solution (a) to 50.0 mL with mobile phase A.
Reference solution (a): Dilute 1.0 mL of test solution (a) to 100.0 mL with mobile phase A. Dilute 1.0 mL of this solution to 10.0 mL with mobile phase A.
Reference solution (b): Dilute 2 mL of test solution (a) to 100 mL with mobile phase A (solution A). Dissolve 5 mg of labetalol impurity A CRS in 50 mL of mobile phase B and dilute to 100 mL with solution A.
Reference solution (c): Dissolve 25.0 mg of labetalol hydrochloride CRS in mobile phase A and dilute to 10.0 mL with mobile phase A. Dilute 1.0 mL of the solution to 50.0 mL with mobile phase A.
Column:
— size: l = 0.15 m, Ø = 4.6 mm;
— stationary phase: end-capped ethylene-bridged octadecylsilyl silica gel for chromatography (hybrid material) R (3.5 μm);
— temperature: 40 °C.
Mobile phase:
— mobile phase A: phosphoric acid R, water for chromatography R (0.1:99.9 V/V);
— mobile phase B: acetonitrile for chromatography R, mobile phase A (50:50 V/V);
| Time
(min) |
Mobile phase A
(per cent V/V) |
Mobile phase B
(per cent V/V) |
| 0 – 5 | 100 | 0 |
| 5 – 40 | 100 → 0 | 0 → 100 |
| 40 – 45 | 0 | 100 |
Flow rate: 1.5 mL/min.
Detection: Spectrophotometer at 230 nm.
Injection: 20 μL of test solution (a) and reference solutions (a) and (b).
Identification of impurities: Use the chromatogram obtained with reference solution (b) to identify the peak due to impurity A.
Relative retention: With reference to labetalol (retention time = about 22 min): impurity A = about 1.1.
System suitability: Reference solution (b):
— resolution: minimum 4.5 between the peaks due to labetalol and impurity A.
Calculation of percentage contents:
— for each impurity, use the concentration of labetalol hydrochloride in reference solution (a).
Limits:
— unspecified impurities: for each impurity, maximum 0.05 per cent;
— total: maximum 0.2 per cent;
— reporting threshold: 0.03 per cent.
Loss on drying (2.2.32)
Maximum 1.0 per cent, determined on 1.000 g by drying in an oven at 105 °C at a pressure not exceeding 0.7 kPa.
Sulfated ash (2.4.14)
Maximum 0.1 per cent, determined on 1.0 g.
ASSAY
Liquid chromatography (2.2.29) as described in the test for related substances with the following modifications.
Mobile phase: Mobile phase A, mobile phase B (45:55 V/V).
Injection: Test solution (b) and reference solution (c).
Run time: Twice the retention time of labetalol.
Retention time: Labetalol = about 2 min.
Calculate the percentage content of C19H25ClN2O3 taking into account the assigned content of labetalol hydrochloride CRS.
IMPURITIES
Other detectable impurities (the following substances would, if present at a sufficient level, be detected by one or other of the tests in the monograph. They are limited by the general acceptance criterion for other/unspecified impurities and/or by the general monograph Substances for pharmaceutical use (2034). It is therefore not necessary to identify these impurities for demonstration of compliance. See also 5.10. Control of impurities in substances for pharmaceutical use) A, B, C, D, E, F, G.
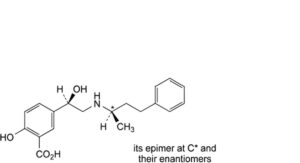
A. mixture of 4 stereoisomers of 2-hydroxy-5-[(1Ξ)-1-hydroxy-2-[[(2Ξ)-4-phenylbutan-2- yl]amino]ethyl]benzoic acid,
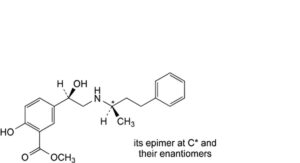
B. mixture of 4 stereoisomers of methyl 2-hydroxy-5-[(1Ξ)-1-hydroxy-2-[[(2Ξ)-4-phenylbutan-2-yl]amino]ethyl]benzoate,
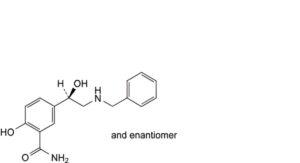
C. 5-[(1RS)-2-(benzylamino)-1-hydroxyethyl]-2-hydroxybenzamide,
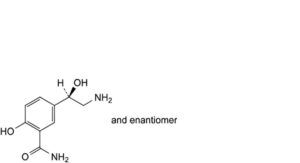
D. 5-[(1RS)-2-amino-1-hydroxyethyl]-2-hydroxybenzamide,
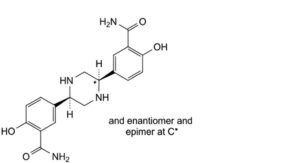
E. mixture of 3 stereoisomers of 5,5′-[(2Ξ,5Ξ)-piperazine-2,5-diyl]bis(2-hydroxybenzamide),
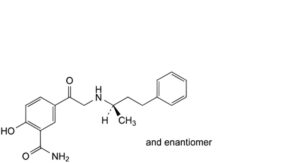
F. 2-hydroxy-5-[2-[[(2RS)-4-phenylbutan-2-yl]amino]acetyl]benzamide,
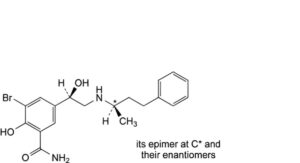
G. mixture of 4 stereoisomers of 3-bromo-2-hydroxy-5-[(1Ξ)-1-hydroxy-2-[[(2Ξ)-4-phenylbutan-2-yl]amino]ethyl]benzamide.