Kanamycin Sulphate
(Kanamycin Monosulfate, Ph. Eur. monograph 0032)
C18H38N4O15S,H2O 601 5965-95-7
Action and use
Aminoglycoside antibacterial.
DEFINITION
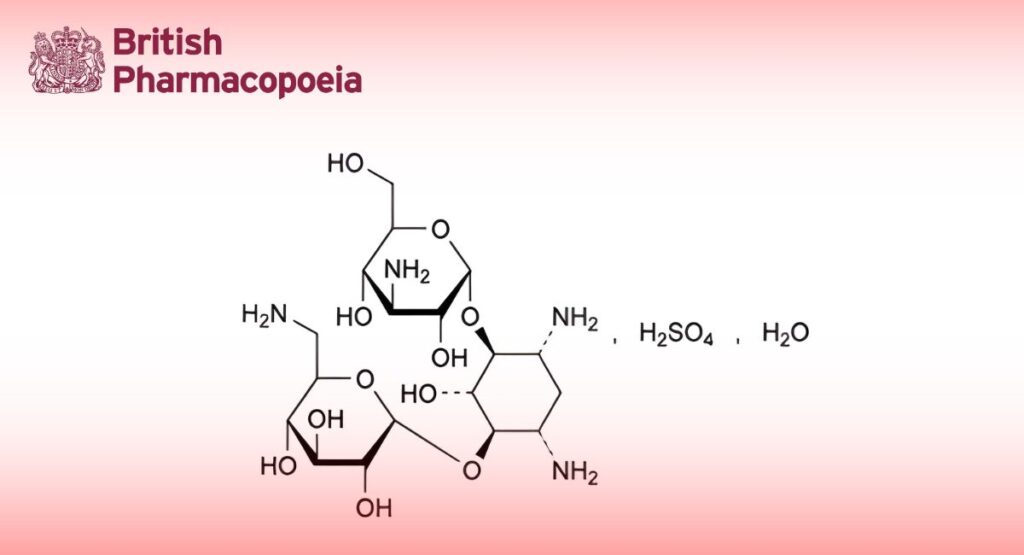
6-O-(3-Amino-3-deoxy-α-D-glucopyranosyl)-4-O-(6-amino-6-deoxy-α-D-glucopyranosyl)-2-deoxy-D-streptamine sulfate monohydrate.
Antimicrobial substance produced by the growth of certain strains of Streptomyces kanamyceticus.
Content
Minimum 750 IU/mg (dried substance).
PRODUCTION
It is produced by methods of manufacture designed to eliminate or minimise substances lowering blood pressure.
CHARACTERS
Appearance
White or almost white, crystalline powder.
Solubility
Freely soluble in water, practically insoluble in acetone and in ethanol (96 per cent).
IDENTIFICATION
A. Thin-layer chromatography (2.2.27).
Test solution: Dissolve 10 mg of the substance to be examined in water R and dilute to 10 mL with the same solvent.
Reference solution (a): Dissolve 10 mg of kanamycin monosulfate CRS in water R and dilute to 10 mL with the same solvent.
Reference solution (b): Dissolve 10 mg of kanamycin monosulfate CRS, 10 mg of neomycin sulfate CRS and 10 mg of streptomycin sulfate for identification CRS in water R and dilute to 10 mL with the same solvent.
Plate: Suitable plate coated with a 0.75 mm layer of a mixture prepared as follows: mix 0.3 g of carbomer R with 240 mL of water R and allow to stand, with moderate shaking, for 1 h; adjust to pH 7 by the gradual addition, with continuous shaking, of dilute sodium hydroxide solution R and add 30 g of silica gel H R.
Pretreatment: Heat the plate at 110 °C for 1 h, allow to cool and use immediately.
Mobile phase: 70 g/L solution of potassium dihydrogen phosphate R.
Application: 10 μL.
Development: Over a path of 12 cm.
Drying: In a current of warm air.
Detection: Spray with a mixture of equal volumes of a 2 g/L solution of 1,3-dihydroxynaphthalene R in ethanol (96 per cent) R and a 460 g/L solution of sulfuric acid R. Heat at 150 °C for 5 min to 10 min.
System suitability: Reference solution (b):
— the chromatogram shows 3 clearly separated spots.
Results: The principal spot in the chromatogram obtained with the test solution is similar in position, colour and size to the principal spot in the chromatogram obtained with reference solution (a).
B. Dissolve 0.5 g in 10 mL of water R. Add 10 mL of picric acid solution R. Initiate crystallisation if necessary by scratching the wall of the tube with a glass rod and allow to stand. Collect the crystals, wash with 20 mL of water R and filter. Dry at 100 °C. The crystals melt (2.2.14) at about 235 °C, with decomposition.
C. Dissolve about 50 mg in 2 mL of water R. Add 1 mL of a 10 g/L solution of ninhydrin R and heat for a few minutes on a water-bath. A violet colour develops.
D. It gives the reactions of sulfates (2.3.1).
TESTS
Solution S
Dissolve 0.20 g in carbon dioxide-free water R and dilute to 20.0 mL with the same solvent.
pH (2.2.3)
6.5 to 8.5 for solution S.
Specific optical rotation (2.2.7)
+ 112 to + 123 (dried substance), determined on solution S.
Kanamycin B
Thin-layer chromatography (2.2.27) as described under Identification A with the following modifications.
Test solution: Dissolve 0.1 g of the substance to be examined in water R and dilute to 20 mL with the same solvent.
Reference solution: Dissolve 4 mg of kanamycin B sulfate CRS in water R and dilute to 20 mL with the same solvent.
Application: 4 μL.
Detection: Spray with ninhydrin and stannous chloride reagent R. Heat at 110 °C for 15 min.
Limit:
— kanamycin B: any spot corresponding to kanamycin B in the chromatogram obtained with the test solution is not more intense than the spot in the chromatogram obtained with the reference solution (4.0 per cent).
Loss on drying (2.2.32)
Maximum 1.5 per cent, determined on 1.000 g by drying at 60 °C at a pressure not exceeding 670 Pa for 3 h.
Sulfated ash (2.4.14)
Maximum 0.5 per cent, determined on 1.0 g.
Sulfate
15.0 per cent to 17.0 per cent of sulfate (dried substance).
Dissolve 0.250 g in 100 mL of water R and adjust the solution to pH 11 with concentrated ammonia R. Add 10.0 mL of 0.1 M barium chloride and about 0.5 mg of phthalein purple R. Titrate with 0.1 M sodium edetate adding 50 mL of ethanol (96 per cent) R when the colour of the solution begins to change and continue the
titration until the violet-blue colour disappears.
1 mL of 0.1 M barium chloride is equivalent to 9.606 mg of SO4.
Pyrogens (2.6.8)
If intended for use in the manufacture of parenteral preparations without a further appropriate procedure for the removal of pyrogens, it complies with the test for pyrogens. Inject per kilogram of the rabbit’s mass 1 mL of a 10 mg/mL solution of the substance to be examined in water for injections R.
ASSAY
Carry out the microbiological assay of antibiotics (2.7.2). Use kanamycin monosulfate CRS as the reference substance.
STORAGE
If the substance is sterile, store in a sterile, tamper-evident container.