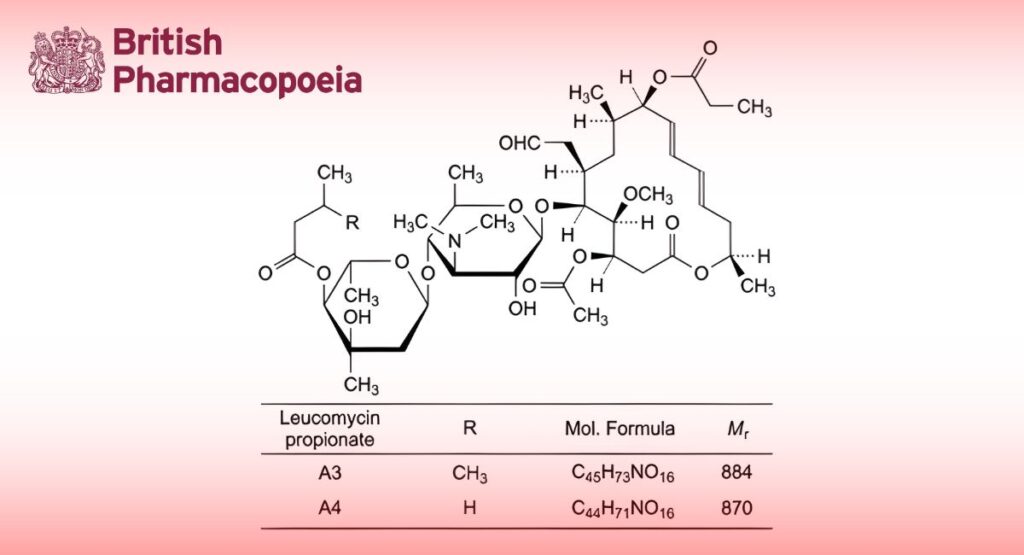
(Ph. Eur. monograph 1982)
Action and use
Antibacterial.
DEFINITION
Propionyl ester of a macrolide antibiotic produced by certain strains of Streptomyces narbonensis var. josamyceticus var. nova, or obtained by any other means. The main component is (4R,5S,6S,7R,9R,10R,11E,13E,16R)-4-(acetyloxy)-6-[[3,6-dideoxy-4-O-[2,6-dideoxy-3-C-methyl-4-O-(3- methylbutanoyl)-α-L-ribo-hexopyranosyl]-3-(dimethylamino)-β-D-glucopyranosyl]oxy]-5-methoxy-9,16- dimethyl-7-(2-oxoethyl)-10-(propanoyloxy)oxacyclohexadeca-11,13-dien-2-one propionate (leucomycin A3 propionate).
Semi-synthetic product derived from a fermentation product.
Content
— minimum 843 Ph. Eur. U./mg (dried substance).
CHARACTERS
Appearance
White or slightly yellowish, crystalline, slightly hygroscopic powder.
Solubility
Practically insoluble in water, freely soluble in methanol and in methylene chloride, soluble in acetone.
IDENTIFICATION
First identification: A, B.
Second identification: B, C.
Prepare solutions in methanol immediately before use.
A. Dissolve 0.10 g in methanol R and dilute to 100.0 mL with the same solvent. Dilute 1.0 mL of the solution to 50.0 mL with methanol R. Examined between 220 nm and 350 nm (2.2.25), the solution shows an absorption maximum at 231 nm. The specific absorbance at the absorption maximum is 310 to 350.
B. Thin-layer chromatography (2.2.27).
Test solution: Dissolve 10 mg of the substance to be examined in methanol R and dilute to 1 mL with the same solvent.
Reference solution (a): Dissolve 10 mg of josamycin propionate CRS in methanol R and dilute to 1 mL with the same solvent.
Reference solution (b); Dissolve 10 mg of josamycin CRS in methanol R and dilute to 1 mL with the same solvent.
Reference solution (c): Dissolve 10 mg of spiramycin CRS in methylene chloride R and dilute to 1 mL with the same solvent.
Reference solution (d): Mix 0.5 mL of reference solution (a) with 0.5 mL of reference solution (b).
Plate: TLC silica gel G plate R.
Mobile phase: methanol R, acetone R, ethyl acetate R, toluene R, hexane R (8:10:20:25:30 V/V/V/V/V).
Application: 10 μL.
Development: Over 2/3 of the plate.
Drying:At 100 °C for 10 min.
Detection: Spray with dilute sulfuric acid R and heat at 100 °C for 10 min.
System suitability: The chromatogram obtained with reference solution (d) shows 2 clearly separated principal spots.
Results: The principal spot in the chromatogram obtained with the test solution is similar in position, colour and size to the principal spot in the chromatogram obtained with reference solution (a) and its position is different from that of the principal spot in the chromatograms obtained with reference solutions (b) and (c).
C. Dissolve about 10 mg in 5 mL of hydrochloric acid R1 and allow to stand for 10-20 min. A pink colour develops, turning brown.
TESTS
Appearance of solution
The solution is clear (2.2.1) and not more intensely coloured than reference solution BY4 (2.2.2, Method II).
Dissolve 1 g in methanol R and dilute to 10 mL with the same solvent.
Specific optical rotation (2.2.7)
-65 to -75 (dried substance).
Dissolve 1.000 g in methanol R and dilute to 100.0 mL with the same solvent. Allow to stand for 30 min before measuring the angle of rotation.
Related substances
Liquid chromatography (2.2.29).
Test solution: Dissolve 50.0 mg of the substance to be examined in acetonitrile for chromatography R and dilute to 100.0 mL with the same solvent.
Reference solution (a): Dissolve 50.0 mg of josamycin propionate CRS in acetonitrile for chromatography R and dilute to 100.0 mL with the same solvent.
Reference solution (b): Dissolve 5 mg of the substance to be examined in 10 mL of methanol R and add 40 μL of dilute phosphoric acid R. Mix, allow to stand for 5 min and inject.
Reference solution (c): Dilute 2.0 mL of reference solution (a) to 100.0 mL with acetonitrile for chromatography R.
Column:
— size: l = 0.15 m, Ø = 3.9 mm;
— stationary phase: end-capped octadecylsilyl silica gel for chromatography R (5 μm);
— temperature: 30 °C.
Mobile phase: acetonitrile R, a 15.4 g/L solution of ammonium acetate R previously adjusted to pH 6.0 with dilute phosphoric acid R (60:40 V/V).
Flow rate: 1.0 mL/min.
Detection: Spectrophotometer at 232 nm.
Injection: 20 μL of the test solution and reference solutions (b) and (c).
Run time: 3 times the retention time of leucomycin A3 propionate.
Relative retention: With reference to leucomycin A3 propionate (retention time = about 18 min): impurity E = about 0.2; impurity A = about 0.3; impurity B = about 0.5; leucomycin A4 propionate = about 0.7; impurity C = about 1.4; impurity D = about 2.0.
System suitability: Reference solution (b):
— resolution: minimum 2.0 between the 2 peaks eluting with a relative retention with reference to leucomycin A3 propionate of about 0.5 and 0.7 respectively.
Limits:
— impurity D: not more than 1.5 times the area of the principal peak in the chromatogram obtained with reference solution (c);
— impurities A, B, C, E: for each impurity, not more than the area of the principal peak in the chromatogram obtained with reference solution (c);
— any other impurity: for each impurity, not more than the area of the principal peak in the chromatogram obtained with reference solution (c);
— total: not more than 7 times the area of the principal peak in the chromatogram obtained with reference solution (c);
— disregard limit: 0.1 times the area of the principal peak in the chromatogram obtained with reference solution (c).
Loss on drying (2.2.32)
Maximum 1.0 per cent, determined on 1.000 g by drying in an oven in vacuo at 60 °C for 3 h.
Sulfated ash (2.4.14)
Maximum 0.2 per cent, determined on 1.0 g.
ASSAY
Dissolve 40.0 mg in 20 mL of methanol R and dilute to 100.0 mL with phosphate buffer solution pH 5.6 R.
Carry out the microbiological assay of antibiotics (2.7.2). Use josamycin propionate CRS as the chemical reference substance.
STORAGE
In an airtight container.
IMPURITIES
Specified impurities A, B, C, D, E.
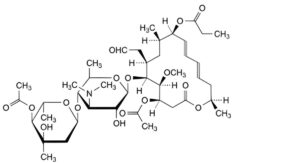
A. leucomycin A8 9-propionate,
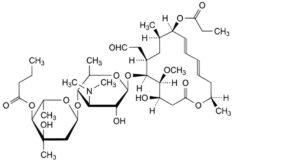
B. leucomycin A5 9-propionate,
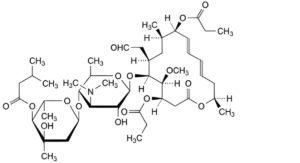
C. platenomycin A1 9-propionate,
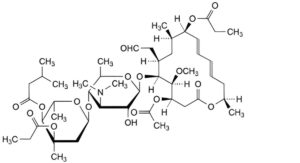
D. leucomycin A3 3′′,9-dipropionate,
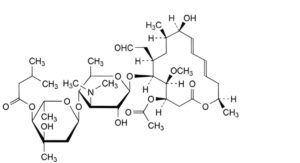
E. (4R,5S,6S,7R,9R,10R,11E,13E,16R)-4-(acetyloxy)-6-[[3,6-dideoxy-4-O-[2,6-dideoxy-3-C-methyl-4-O-(3- methylbutanoyl)-α-L-ribo-hexopyranosyl]-3-(dimethylamino)-β-D-glucopyranosyl]oxy]-10-hydroxy-5-methoxy- 9,16-dimethyl-7-(2-oxoethyl)oxacyclohexadeca-11,13-dien-2-one (josamycin).