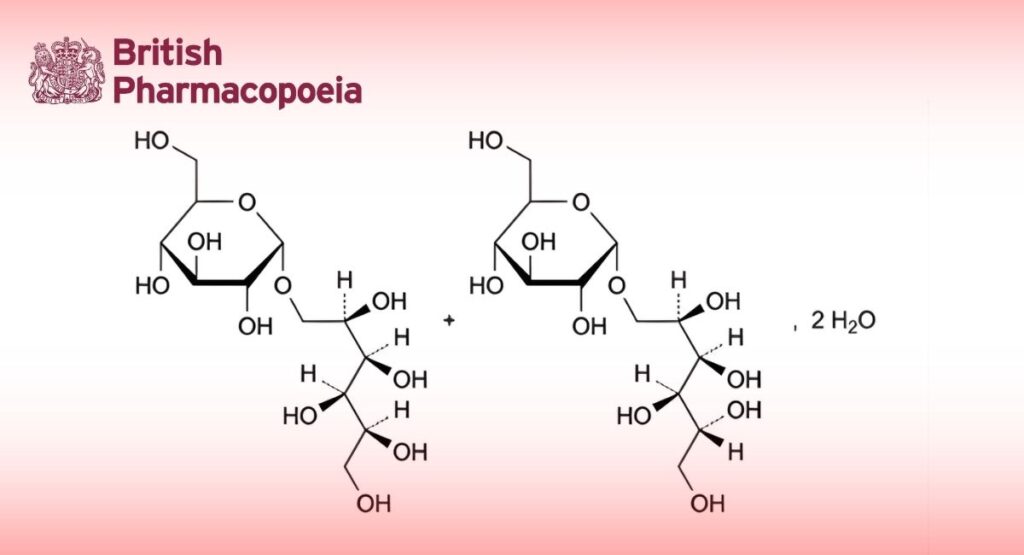
Isomalt1
(Ph. Eur. monograph 1531)
C12H24O11 344.3
C12H24O11,2H2O 380.3
Anhydrous isomalt 64519-82-0
Action and use
Sweetening agent.
DEFINITION
Mixture of 6-O-α-D-glucopyranosyl-D-glucitol (6-O-α-D-glucopyranosyl-D-sorbitol; 1,6-GPS) and 1-O-α-D-glucopyranosyl-D- mannitol (1,1-GPM).
Content
98.0 per cent to 102.0 per cent for the mixture of 1,6-GPS and 1,1-GPM and neither of the 2 components is less than 3.0 per cent (anhydrous substance).
CHARACTERS
Appearance
White or almost white powder or granules.
Solubility
Freely soluble in water, practically insoluble in anhydrous ethanol.♦
IDENTIFICATION
First identification: A.
Second identification: B, C.♢
A. Examine the chromatograms obtained in the assay.
Results: The 2 principal peaks in the chromatogram obtained with the test solution are similar in retention time to the 2 principal peaks in the chromatogram obtained with reference solution (a).
B. Thin-layer chromatography (2.2.27).
Test solution: Dissolve 50 mg of the substance to be examined in water R and dilute to 10 mL with the same solvent.
Reference solution: Dissolve 50 mg of isomalt CRS in water R and dilute to 10 mL with the same solvent.
Plate: TLC silica gel F254 plate R.
Mobile phase: acetic acid R, propionic acid R, water R, ethyl acetate R, pyridine R (5:5:10:50:50 V/V/V/V/V).
Application: 1 μL; thoroughly dry the points of application in warm air.
Development: Over 1/2 of the plate.
Drying: In a current of warm air.
Detection: Dip for 3 s in a 1 g/L solution of sodium periodate R and dry in a current of hot air; dip for 3 s in a mixture of volume of acetic acid R, 1 volume of anisaldehyde R, 5 volumes of sulfuric acid R and 90 volumes of anhydrous ethanol R; dry in a current of hot air until coloured spots become visible; the background colour may be brightened in
warm steam; examine in daylight.
Results: The chromatogram obtained with the reference solution shows 2 blue-grey spots with RF values of about 0.13 (1,6-GPS) and 0.16 (1,1-GPM). The chromatogram obtained with the test solution shows principal spots similar in position and colour to the principal spots in the chromatogram obtained with the reference solution.
C. To 3 mL of a freshly prepared 100 g/L solution of pyrocatechol R add 6 mL of sulfuric acid R while cooling in iced water. To 3 mL of the cooled mixture add 0.3 mL of a 100 g/L solution of the substance to be examined. Heat gently over a naked flame for about 30 s. A pink colour develops.♢
TESTS
Conductivity (2.2.38)
Maximum 20 μS·cm .
Dissolve 20.0 g in carbon dioxide-free water R with gentle heating (40-50 °C) and dilute to 100.0 mL with the same solvent. Measure the conductivity of the solution while gently stirring with a magnetic stirrer.
Reducing sugars
Maximum 0.3 per cent, expressed as glucose equivalent.
Dissolve 3.3 g in 10 mL of water R with gentle heating. Cool and add 20 mL of cupri-citric solution R and a few glass beads. Heat so that boiling begins after 4 min and maintain boiling for 3 min. Cool rapidly and add 100 mL of a 2.4 per cent V/V solution of glacial acetic acid R and 20.0 mL of 0.025 M iodine. With continuous shaking, add 25 mL of a mixture of 6 volumes of hydrochloric acid R and 94 volumes of water R. When the precipitate has dissolved, titrate the excess of iodine with 0.05 M sodium thiosulfate using 1 mL of starch solution R as indicator, added towards the end of the titration.
Not less than 12.8 mL of 0.05 M sodium thiosulfate is required.
Related substances
Liquid chromatography (2.2.29).
Test solution: Dissolve 0.200 g of the substance to be examined in 4 mL of water R and dilute to 10.0 mL with the same solvent.
Reference solution (a): Dissolve 0.200 g of isomalt CRS in 4 mL of water R and dilute to 10.0 mL with the same solvent.
Reference solution (b): Dissolve 10.0 mg of sorbitol CRS (impurity C) and 10.0 mg of mannitol CRS (impurity B) in 20 mL of water R and dilute to 100.0 mL with the same solvent.
Precolumn:
— size: l = 30 mm, Ø = 4.6 mm;
— stationary phase: strong cation-exchange resin (calcium form) R (9 μm);
— temperature: 80 ± 3 °C.
Column:
— size: l = 0.3 m, Ø = 7.8 mm;
— stationary phase: strong cation-exchange resin (calcium form) R (9 μm);
— temperature: 80 ± 3 °C.
Mobile phase: Degassed water for chromatography R.
Flow rate: 0.5 mL/min.
Detection Differential refractometer maintained at a constant temperature (e.g. 40 °C).
Injection: 20 μL.
Run time: 2.5 times the retention time of 1,1-GPM.
Relative retention: With reference to 1,1-GPM (retention time = about 14 min): 1,6-GPS = about 1.2; impurity B = about 1.6; impurity C = about 2.0.
System suitability: Reference solution (a):
— resolution: minimum 2.0 between the peaks due to 1,1-GPM and 1,6-GPS.
Limits:
— impurities B, C: for each impurity, not more than the area of the corresponding peak in the chromatogram obtained with reference solution (b) (0.5 per cent);
— unspecified impurities: for each impurity, not more than the area of the peak due to impurity C in the chromatogram obtained with reference solution (b) (0.5 per cent);
— total: not more than 4 times the area of the peak due to impurity C in the chromatogram obtained with reference solution (b) (2.0 per cent);
— disregard limit: 0.2 times the area of the peak due to impurity C in the chromatogram obtained with reference solution (b) (0.1 per cent).
Water (2.5.12)
Maximum 7.0 per cent, determined on 0.300 g. As solvent, use a mixture of 20 mL of anhydrous methanol R and 20 mL of formamide R1 at 50 ± 5 °C.
ASSAY
Liquid chromatography (2.2.29) as described in the test for related substances with the following modification
Injection: Test solution and reference solution (a).
Calculate the percentage content of isomalt (1,1-GPM and 1,6-GPS) taking into account the assigned contents of 1,1-GPM and 1,6-GPS in isomalt CRS.
LABELLING
The label states the percentage contents of 1,1-GPM and 1,6-GPS.
IMPURITIES
Specified impurities B, C.
Other detectable impurities (the following substances would, if present at a sufficient level, be detected by one or other of the tests in the monograph. They are limited by the general acceptance criterion for other/unspecified impurities. It is therefore not necessary to identify these impurities for demonstration of compliance. See also 5.10. Control of impurities in substances for pharmaceutical use) A, D.
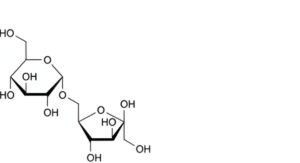
A. 6-O-α-D-glucopyranosyl-β-D-arabino-hex-2-ulofuranose (isomaltulose),
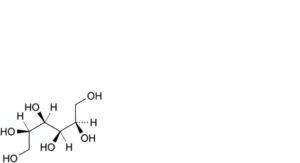
B. D-mannitol,
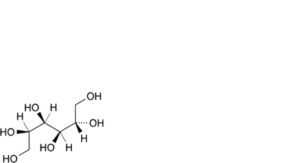
C. D-glucitol (D-sorbitol),
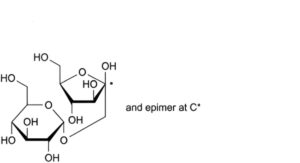
D. 1-O-α-D-glucopyranosyl-D-arabino-hex-2-ulofuranose (trehalulose).
1 This monograph has undergone pharmacopoeial harmonisation. See chapter 5.8 Pharmacopoeial harmonisation.