Edition: BP 2025 (Ph. Eur. 11.6 update)
General Notices
Action and use
Treatment of iron-deficiency anaemia.
DEFINITION
Iron Sucrose Injection is a sterile colloidal solution containing a complex of iron(III) hydroxide with sucrose of average molecular weight between 34000 and 60000.
PRODUCTION
Iron Sucrose Injection is produced by a method of manufacture designed to provide an iron-sucrose complex with appropriate iron absorption characteristics. This may be confirmed for routine control purposes by the use of an appropriate combination of physico-chemical tests, subject to the agreement of the competent authority.
A suitable test is carried out to demonstrate (1) the amount of iron(II) present in the injection is not more than 0.4% w/v of the total iron content and (2) there are no low molecular weight complexes in the injection.
The injection complies with the requirements stated under Parenteral Preparations and with the following requirements.
Content of iron, Fe
95.0 to 105.0% of the stated amount.
Content of sucrose
90.0 to 110.0% of the stated amount.
IDENTIFICATION
A. To a quantity of the injection containing the equivalent of 20 mg of iron, add 20 mL of water and 5 mL of hydrochloric acid and boil for 5 minutes. Cool, add an excess of 13.5M ammonia and filter. Wash the precipitate with water, dissolve in the minimum volume of 2M hydrochloric acid and add sufficient water to produce 20 mL. The resulting solution yields reaction B characteristic of iron salts, Appendix VI.
B. In the Assay for Sucrose, the retention time of the principal peak in the chromatogram obtained with solution (1) corresponds to that of the principal peak in the chromatogram obtained with solution (2).
C. Complies with the test for Molecular weight determination.
TESTS
Alkalinity
pH, 10.5 to 11.0, Appendix V L.
Osmolality
The osmolality, Appendix V N, of the injection is 1150 mosmol/kg to 1350 mosmol/kg.
Clarity of solution
Appendix IV A. To a quantity of the injection containing 10 mg of iron, add 100 mL of water and adjust to pH 6.0 with 0.1M hydrochloric acid. The solution must not have any turbidity. Add 0.1M hydrochloric acid dropwise until a faint turbidity develops, the pH of the solution is between 4.4 and 5.3.
Molecular weight determination
Carry out the method for size-exclusion chromatography, Appendix III C, using the following solutions in the mobile phase.
(1) Dilute a quantity of the injection with a sufficient volume of the mobile phase to produce a solution expected to contain 1% w/v of iron.
(2) 0.4% w/v of polysaccharide molecular weight standard 5000 Da. Allow to stand for a minimum of 12 hours and gently swirl to dissolve any agglomerated particles.
(3) 0.4% w/v of polysaccharide molecular weight standard 25000 Da. Allow to stand for a minimum of 12 hours and gently swirl to dissolve any agglomerated particles.
(4) 0.4% w/v of polysaccharide molecular weight standard 50000 Da. Allow to stand for a minimum of 12 hours and gently swirl to dissolve any agglomerated particles.
(5) 0.4% w/v of polysaccharide molecular weight standard 150000 Da. Allow to stand for a minimum of 12 hours and gently swirl to dissolve any agglomerated particles.
(6) 0.4% w/v of polysaccharide molecular weight standard 270000 Da. Allow to stand for a minimum of 12 hours and gently swirl to dissolve any agglomerated particles.
(7) 0.4% w/v of polysaccharide molecular weight standard 410000 Da. Allow to stand for a minimum of 12 hours and gently swirl to dissolve any agglomerated particles.
(8) 0.4% w/v of polysaccharide molecular weight standards 12000 Da. Allow to stand for a minimum of 12 hours and gently swirl to dissolve any agglomerated particles.
CHROMATOGRAPHIC CONDITIONS
(a) Use two columns (30 cm X 7.8 mm) packed with hydrophilic polyhydroxymethacrylate gel of totally porous spherical resin for chromatography, connected in series with pore sizes of 1000Â and 120Â respectively.
(b) Use isocratic elution and the mobile phase described below.
(c) Use a flow rate of 0.5 mL per minute.
(d) Use a column temperature of 45°.
(e) Use a refractive index detector, maintained at 45°.
(f) Inject 25 pL of each solution.
MOBILE PHASE
A solution containing 0.02% w/v of sodium azide, 0.276% w/v of sodium dihydrogen orthophosphate monohydrate and 0.356% w/v of disodium hydrogen orthophosphate dihydrate in water. Adjust the pH to 6.8 with orthophosphoric acid.
SYSTEM SUITABILITY
The test is not valid unless:
the correlation coefficient of the calculated calibration curve is at least 0.98;
in the chromatogram obtained with solution (8), the calculated molecular weight is concordant with the value supplied with polysaccharide molecular weight standard 12000 Da.
DETERMINATION OF MOLECULAR WEIGHT
Determine the peak areas and retention times of the principal peaks in solutions (2), (3), (4), (5) (6) and (7). Plot the peak area for each solution as a function of concentration and peak area to create a cubic calibration curve.
From the calibration curve obtained with solutions (2) to (7), calculate the molecular weight of the complex in the injection.
The molecular weight distribution curve of solution (1) is split into fractions correlating to the molecular weight of solutions
(2) to (7). Calculate:
the weight-average molecular weight (MW):
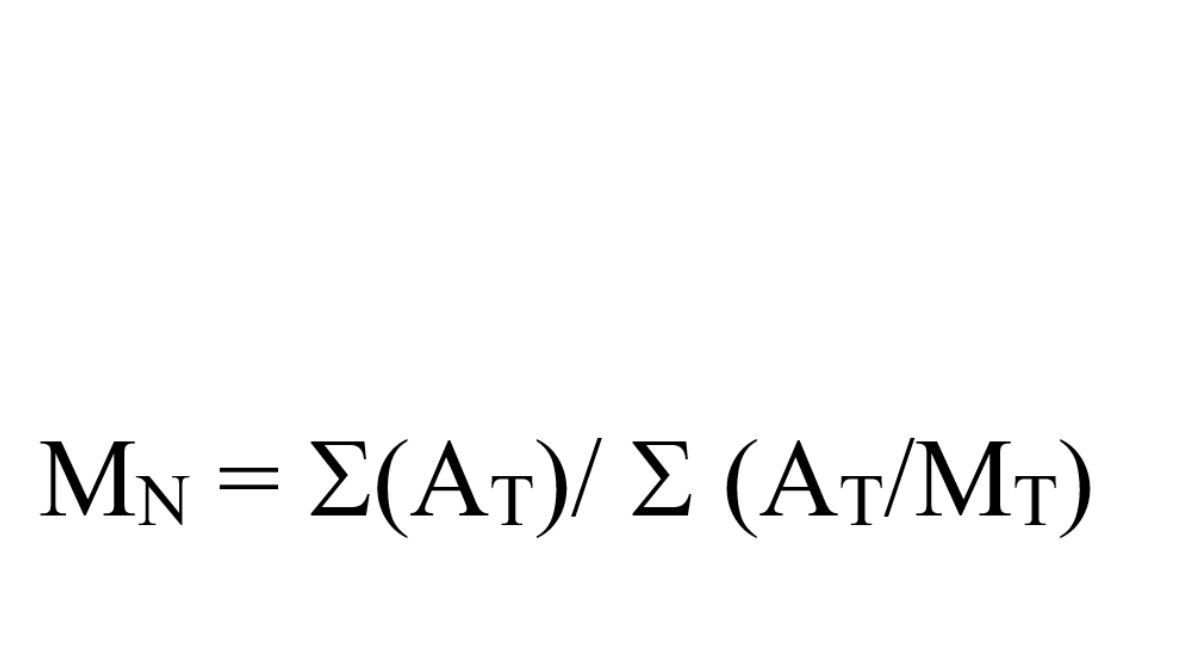
and the number-average molecular weight (MN):
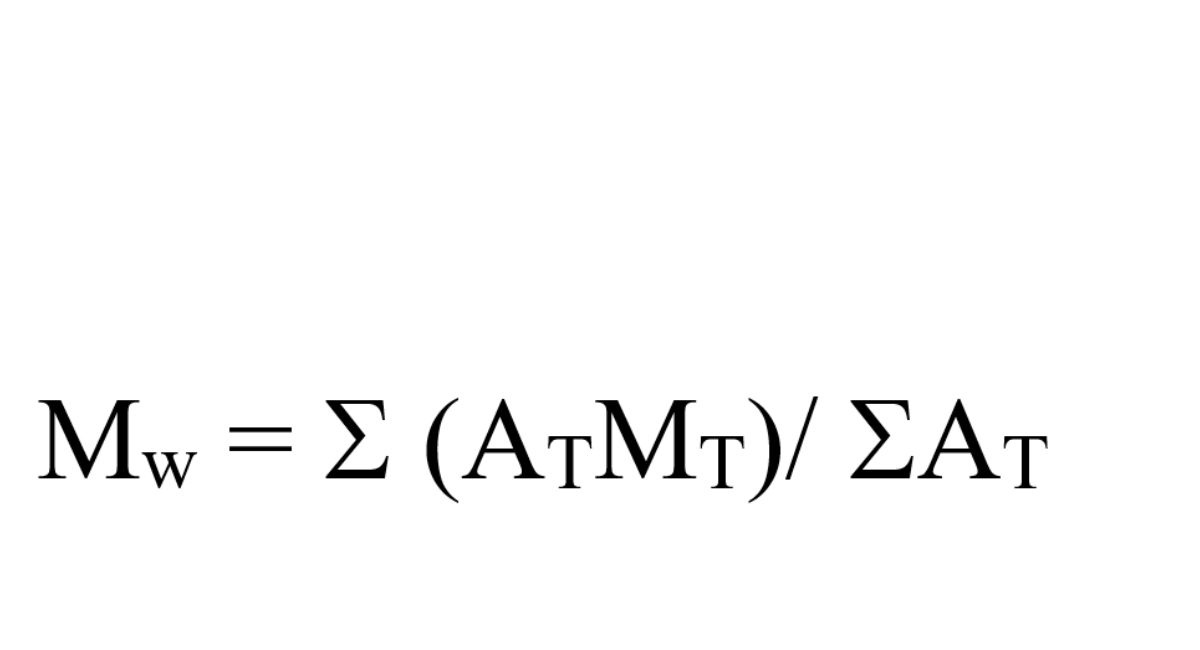
where
AT = the area of each fraction of the sample distribution
MT = the corresponding mean molecular weight of each fraction as determined from its retention time on the calibration curve.
The molecular weight distribution curve obtained for the Injection conforms to the following parameters:
MW = 34,000 to 60,000 Da
MN = Not less than 24,000 Da
MW/MN = Not more than 1.7.
ASSAY
For iron
To a quantity of the injection containing the equivalent of 100 mg of iron add 100 mL of 0.5M hydrochloric acid and 10 mL of acetic acid. Adjust the pH to 2.4 using dilute sodium hydroxide, add 0.5 mL of hydrogen peroxide solution (20 vol) and 2.5 mL of tiron indicator solution. Titrate with 0.1M sodium edetate VS until the dark green colour changes to yellow and remains yellow for at least one minute. Each mL of 0.1M sodium edetate VS is equivalent to 5.585 mg of iron.
For sucrose
Carry out the method for liquid chromatography, Appendix III D, using the following solutions.
(1) To a volume of the injection containing 40mg of Iron Sucrose add 2.5 mL of a 3.0% w/v solution of sodium dihydrogen orthophosphate monohydrate, mix and allow to stand for 10 minutes, add sufficient water to produce 25 mL, centrifuge and filter discarding the first 2 mL of filtrate.
(2) 1.30% w/v of sucrose BPCRS in mobile phase.
(3) 1.60% w/v of sucrose BPCRS in mobile phase.
(4) 1.80% w/v of sucrose BPCRS in mobile phase.
(5) 2.10% w/v of sucrose BPCRS in mobile phase.
(6) 2.30% w/v of sucrose BPCRS in mobile phase.
CHROMATOGRAPHIC CONDITIONS
(a) Use a stainless steel column (25 cm X 4 mm) packed with aminopropylsilyl silica gel for chromatography (3-10 pm).
(b) Use isocratic elution and the mobile phase described below.
(c) Use a flow rate of 2 mL per minute.
(d) Use an ambient column temperature.
(e) Use a refractive index detector.
(f) Inject 20 pL of each solution.
MOBILE PHASE
21 volumes of water and 79 volumes of acetonitrile.
DETERMINATION OF CONTENT
Determine the peak areas of the principal peak in solutions (2), (3), (4), (5) and (6), plot the peak area for each solution as a function of concentration of sucrose and draw a straight line best fitting the five plotted points. From the graph obtained calculate the content of sucrose in the injection using the declared content of sucrose in sucrose BPCRS.
LABELLING
The strength is stated as the equivalent amount of iron, Fe, in a suitable dose-volume.






