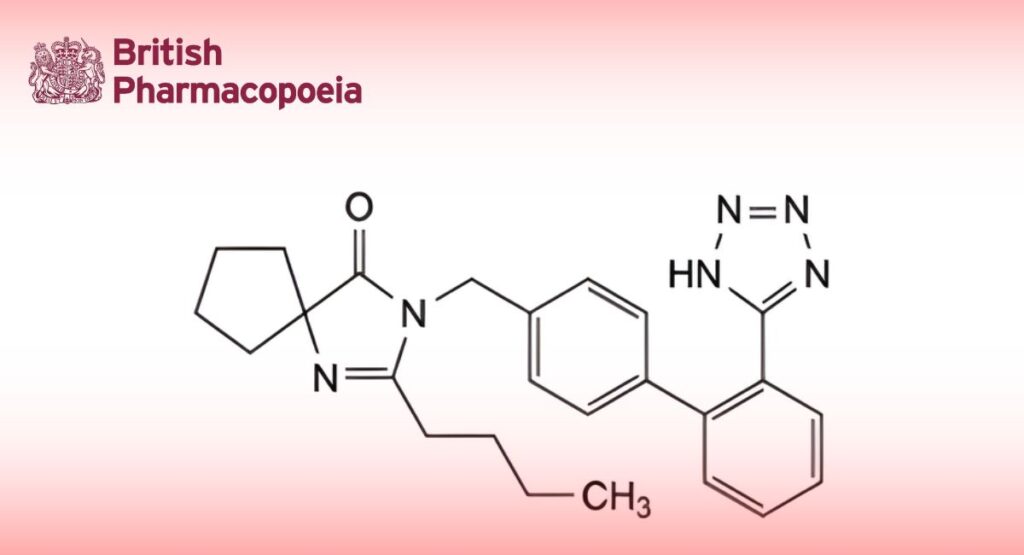
(Ph. Eur. monograph 2465)
C25H28N6O 428.5 138402-11-6
Action and use
Angiotensis II (AT1) receptor antagonist.
Preparation
Irbesartan Tablets
DEFINITION
2-Butyl-3-[[2′-(1H-tetrazol-5-yl)[1,1′-biphenyl]-4-yl]methyl]-1,3-diazaspiro[4.4]non-1-en-4-one.
Content
99.0 per cent to 101.0 per cent (anhydrous substance).
CHARACTERS
Appearance
White or almost white, crystalline powder.
Solubility
Practically insoluble in water, sparingly soluble in methanol, slightly soluble in methylene chloride.
It shows polymorphism (5.9).
IDENTIFICATION
Infrared absorption spectrophotometry (2.2.24).
Comparison: irbesartan CRS.
If the spectra obtained in the solid state show differences, dissolve the substance to be examined and the reference substance separately in methanol R, evaporate to dryness at 60 °C and record new spectra using the residues.
TESTS
Appearance of solution
The solution is clear (2.2.1) and not more intensely coloured than reference solution B7 (2.2.2, Method II).
Dissolve 0.50 g in a mixture of 1 volume of 2 M sodium hydroxide R and 9 volumes of methanol R2 and dilute to 10 mL with the same mixture of solvents.
Impurity B
Liquid chromatography (2.2.29). Prepare the solutions immediately before use.
Test solution: Dissolve 0.100 g of the substance to be examined in the mobile phase and dilute to 5.0 mL with the mobile phase.
Reference solution: Dissolve 25.0 mg of sodium azide R (sodium salt of impurity B) in the mobile phase and dilute to 100.0 mL with the mobile phase. Dilute 0.25 mL of the solution to 200.0 mL with the mobile phase.
Precolumn (used to prevent saturation of the column with irbesartan):
— size: l = 0.05 m, Ø = 4 mm;
— stationary phase: strongly basic anion-exchange resin for chromatography R (8.5 μm).
Column:
— size: l = 0.25 m, Ø = 4 mm;
— stationary phase: strongly basic anion-exchange resin for chromatography R (8.5 μm).
Mobile phase: 4.2 g/L solution of sodium hydroxide R in carbon dioxide-free water R.
Flow rate: 1.0 mL/min.
Detection: Conductivity detector with a sensitivity of 3 μS; use a self-regenerating anion suppressor.
Neutralisation of the eluent: Either chemical or electrochemical:
— chemical: by continuous countercurrent circulation in a neutralising micromembrane, performed before detection:
— neutralising solvent: 0.025 M sulfuric acid;
— flow rate: 10 mL/min;
— pressure: about 100 kPa.
— electrochemical: 300 mA (for example).
Injection 200 μL; after each injection of the test solution, rinse the precolumn with a mixture of mobile phase and methanol R (40:60 V/V) for 10 min; equilibrate to initial conditions as necessary; a switch valve can be used to avoid disconnecting the precolumn from the column.
Run time: 25 min.
Retention time: Impurity B = about 14 min.
System suitability: Reference solution:
— signal-to-noise ratio: minimum 10 for the peak due to impurity B.
Limit:
— impurity B: not more than the area of the corresponding peak in the chromatogram obtained with the reference solution (10 ppm).
Related substances
Liquid chromatography (2.2.29).
Buffer solution pH 3.2 Mix 5.5 mL of phosphoric acid R and 950 mL of water for chromatography R and adjust to pH 3.2 with triethylamine R.
Test solution: Dissolve 50 mg of the substance to be examined in methanol R2 and dilute to 50.0 mL with the same solvent.
Reference solution (a): Dilute 1.0 mL of the test solution to 100.0 mL with methanol R2. Dilute 1.0 mL of this solution to 10.0 mL with methanol R2.
Reference solution (b): Dissolve 5 mg of the substance to be examined and 5 mg of irbesartan impurity A CRS inmethanol R2 and dilute to 10 mL with the same solvent. Dilute 1 mL of the solution to 10 mL with methanol R2.
Column:
— size: l = 0.25 m, Ø = 4 mm;
— stationary phase: end-capped octadecylsilyl silica gel for chromatography R (5 μm).
Mobile phase: acetonitrile R1, buffer solution pH 3.2 (33:67 V/V).
Flow rate: 1.0 mL/min.
Detection: Spectrophotometer at 220 nm.
Injection: 10 μL.
Run time: 1.4 times the retention time of irbesartan.
Identification of impurities: Use the chromatogram obtained with reference solution (b) to identify the peak due to impurity A.
Relative retention: With reference to irbesartan (retention time = about 23 min): impurity A = about 0.7.
System suitability: Reference solution (b):
— resolution: minimum 3.0 between the peaks due to impurity A and irbesartan.
Limits:
— impurity A: not more than 1.5 times the area of the principal peak in the chromatogram obtained with reference solution (a) (0.15 per cent);
— unspecified impurities: for each impurity, not more than the area of the principal peak in the chromatogram obtained with reference solution (a) (0.10 per cent);
— total: not more than twice the area of the principal peak in the chromatogram obtained with reference solution (a) (0.2 per cent);
— disregard limit: 0.5 times the area of the principal peak in the chromatogram obtained with reference solution (a) (0.05 per cent).
Water (2.5.12)
Maximum 0.5 per cent, determined on 1.00 g.
Sulfated ash (2.4.14)
Maximum 0.1 per cent, determined on 1.0 g.
ASSAY
Dissolve 0.300 g in 50 mL of anhydrous acetic acid R. Titrate with 0.1 M perchloric acid, determining the end-point potentiometrically (2.2.20).
1 mL of 0.1 M perchloric acid is equivalent to 42.85 mg of C25H28N6O.
IMPURITIES
Specified impurities A, B.
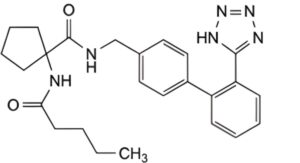
A. 1-(pentanoylamino)-N-[[2′-(1H-tetrazol-5-yl)[1,1′-biphenyl]-4-yl]methyl]cyclopentane-1-carboxamide,
B. N3– : trinitride (azide).