Edition: BP 2025 (Ph. Eur. 11.6 update)
Action and use
Anticholinergic (antimuscarinic) bronchodilator.
DEFINITION
Ipratropium Nebuliser Solution is a solution of Ipratropium Bromide in Water for Injections and may contain sodium chloride.
The nebuliser solution complies with the requirements stated under Preparations for Inhalation and with the following requirements.
Content of ipratropium bromide, C20H30NO3Br,H2O
95.0 to 110.0% of the stated amount.
IDENTIFICATION
Evaporate a volume of the nebuliser solution containing 1.5 mg of Ipratropium Bromide to dryness on a water bath. Shake the residue with 5 mL of methanol and filter (Whatman GF/C is suitable). Evaporate the filtrate to dryness on a water bath and dry the residue at room temperature at a pressure of 1 kPa for 15 minutes. The infrared absorption spectrum of the
dried residue, Appendix II A, is concordant with a spectrum prepared from a mixture of 5 mg of ipratropium bromide BPCRS and 45 mg of sodium chloride dissolved in the minimum quantity of water, evaporated to dryness on a water bath and treated in a similar manner to the substance being examined beginning at the words “Shake the residue with 5 mL of methanol,…”.
TESTS
Acidity
pH, 3.0 to 4.0, Appendix V L.
Related substances
Carry out the method for liquid chromatography, Appendix III D, using the following solutions.
(1) Dilute a quantity of the nebuliser solution, if necessary, with sufficient 0.001M hydrochloric acid to produce a solution containing 0.02% w/v of Ipratropium Bromide.
(2) Dilute 1 volume of solution (1) to 200 volumes with 0.001M hydrochloric acid.
(3) 0.005% w/v of ipratropium bromide impurity B EPCRS and 0.005% w/v of ipratropium bromide BPCRS in 0.001M hydrochloric acid.
(4) Dilute 1 volume of solution (2) to 5 volumes with 0.001M hydrochloric acid.
CHROMATOGRAPHIC CONDITIONS
(a) Stainless steel column (12.5 cm × 4.6 mm) packed with octylsilyl silica gel for chromatography (5 μm) (Columbus C8 is suitable).
(b) Use isocratic elution using the mobile phase described below.
(c) Use a flow rate of 0.5 mL per minute.
(d) Use an ambient column temperature.
(e) Detection wavelength of 210 nm.
(f) Inject 20 μL of each solution.
(g) For solution (1) allow the chromatography to proceed for 6 times the retention time of ipratropium.
MOBILE PHASE
A mixture of 4 volumes of freshly distilled triethylamine, 50 volumes of propan-2-ol R1, 100 volumes of acetonitrile R1 and 850 volumes of a 0.1% w/v solution of sodium methanesulfonate adjusted to pH 3.0 with orthophosphoric acid.
When the chromatograms are recorded under the prescribed conditions, the retention time of ipratropium is about 11 minutes.
SYSTEM SUITABILITY
The test is not valid unless, in the chromatogram obtained with solution (3), the resolution between the peaks due to ipratropium bromide and impurity B is at least 1.2.
LIMITS
In the chromatogram obtained with solution (1):
the area of any secondary peak is not greater than the area of the principal peak in the chromatogram obtained with solution (2) (0.5%);
the sum of the areas of any such peaks is not greater than 3 times the area of the principal peak in the chromatogram obtained with solution (2) (1.5%).
Disregard any peak with an area less than the area of the principal peak in the chromatogram obtained with solution (4) (0.1%).
ASSAY
Carry out the method for liquid chromatography, Appendix III D, using the following solutions.
(1) Dilute a quantity of the nebuliser solution, if necessary, with sufficient 0.001M hydrochloric acid to produce a solution containing 0.02% w/v of Ipratropium Bromide.
(2) 0.02% w/v of ipratropium bromide BPCRS in 0.001M hydrochloric acid.
(3) 0.005% w/v of ipratropium bromide impurity B EPCRS and 0.005% w/v of ipratropium bromide BPCRS in 0.001M hydrochloric acid.
CHROMATOGRAPHIC CONDITIONS
The chromatographic procedure described under Related substances may be used.
SYSTEM SUITABILITY
The test is not valid unless, in the chromatogram obtained with solution (3), the resolution between the two principal peaks is at least 1.2.
DETERMINATION OF CONTENT
Calculate the content of C20H30NO3Br,H2O in the solution using the declared content of C20H30NO3Br,H2O in ipratropium bromide BPCRS.
STORAGE
Ipratropium Nebuliser Solution should be stored protected from light in a sealed container.
IMPURITIES
The impurities limited by the requirements of this monograph include.
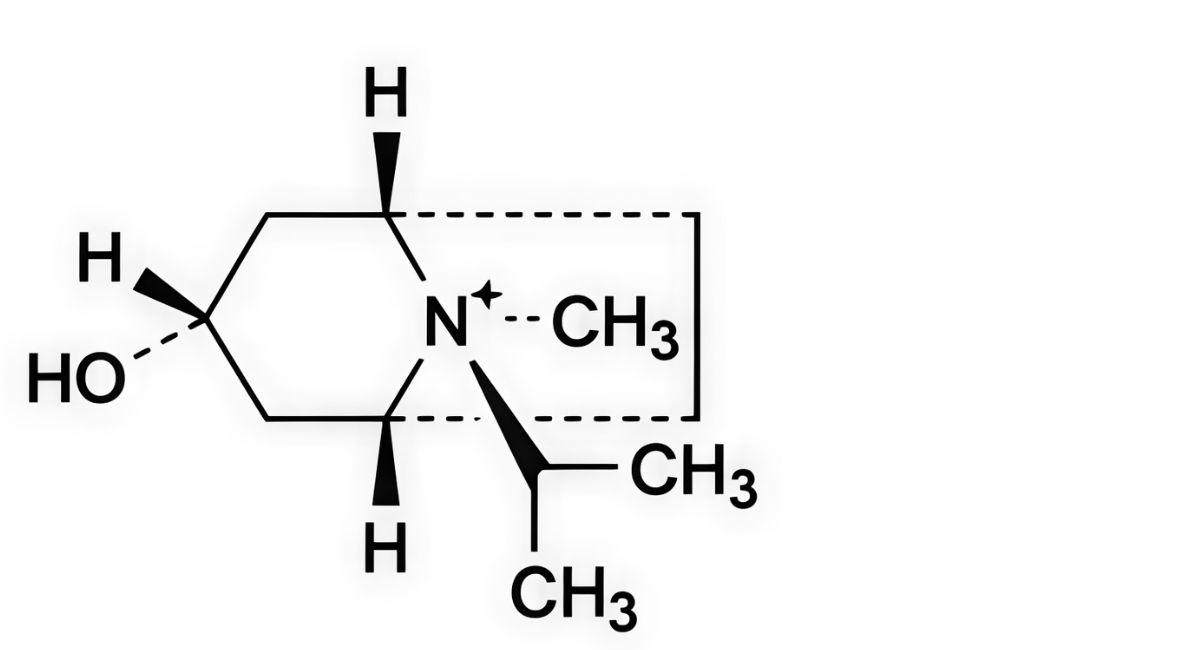
A. (1R,3r,5S,8r)-3-hydroxy-8-methyl-8-(1-methylethyl)-8-azoniabicyclo[3.2.1]octane,
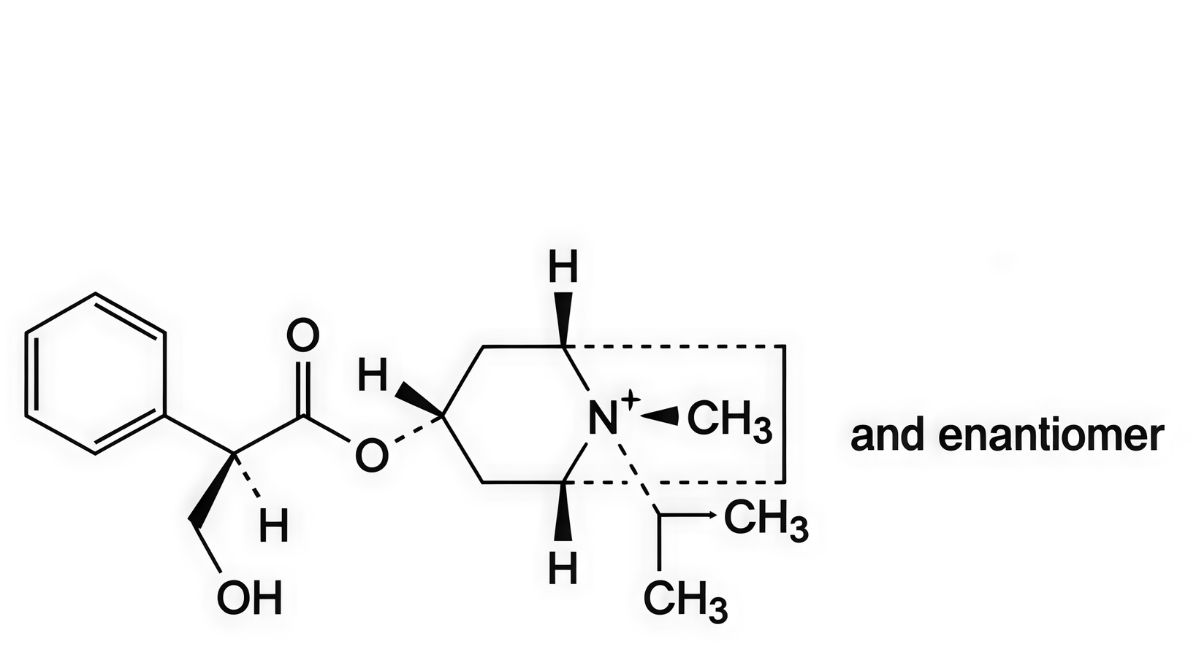
B. (1R,3r,5S,8s)-3-[[(2RS)-3-hydroxy-2-phenylpropanoyl]oxy]-8-methyl-8-(1-methylethyl)-8- azoniabicyclo[3.2.1]octane,
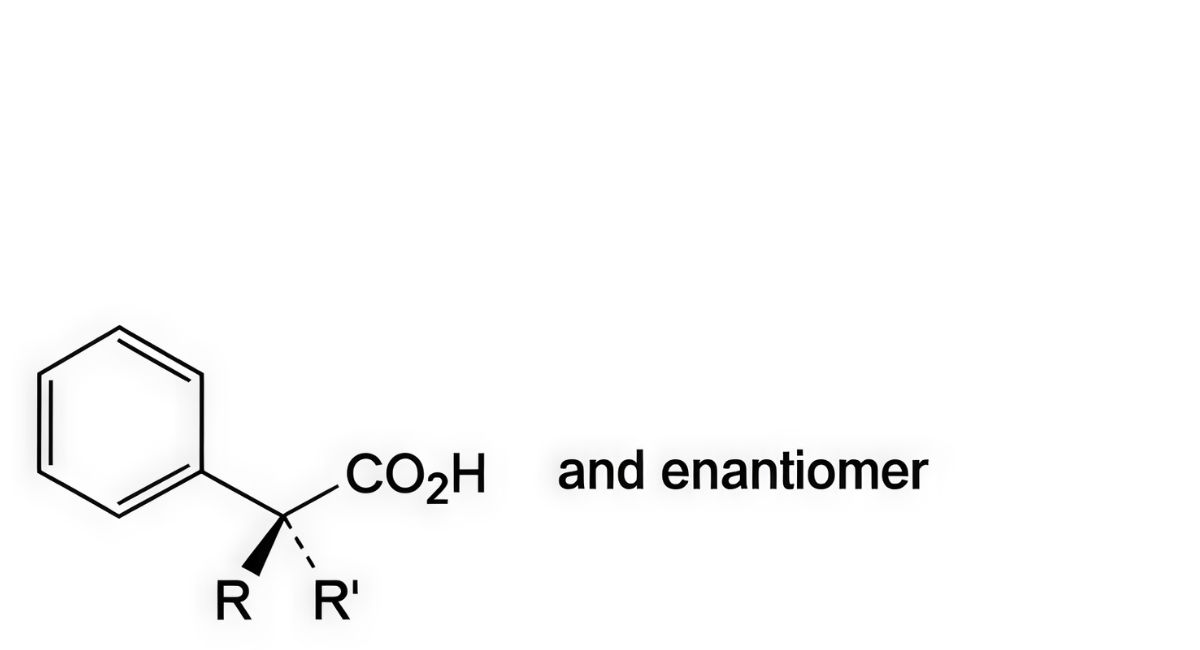
C. R = CH2-OH, R′ = H: (2RS)-3-hydroxy-2-phenylpropanoic acid (DL-tropic acid),
D. R + R′ = CH2: 2-phenylpropenoic acid (atropic acid).






