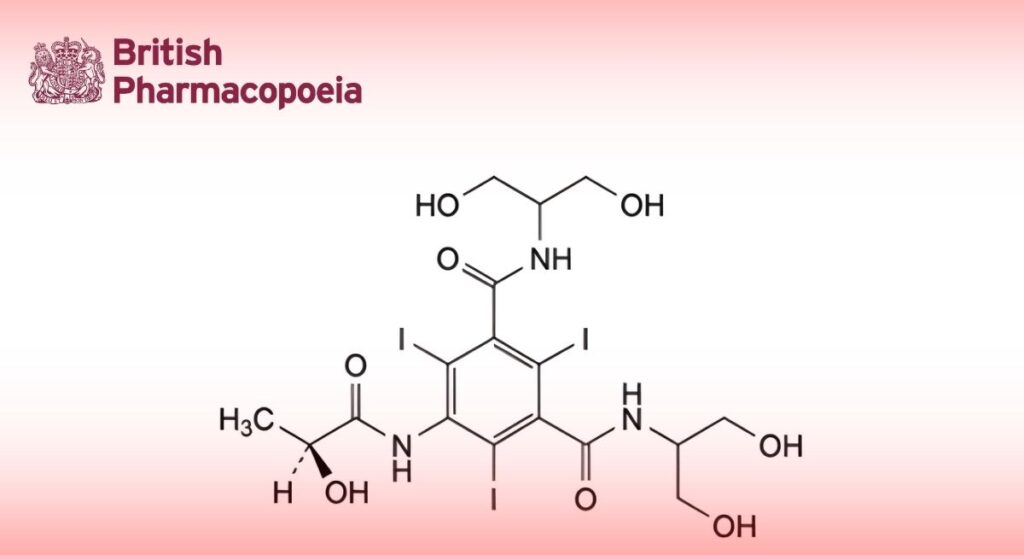
(Ph. Eur. monograph 1115)
C17H22I3N3O8 777 60166-93-0
Action and use
Iodinated contrast medium.
Preparations
Iopamidol Injection
Iopamidol Oral Solution
DEFINITION
N,N′-Bis[2-hydroxy-1-(hydroxymethyl)ethyl]-5-[[(2S)-2-hydroxypropanoyl]amino]-2,4,6-triiodobenzene-1,3-dicarboxamide.
Content
98.5 per cent to 101.0 per cent (dried substance).
CHARACTERS
Appearance
White or almost white powder.
Solubility
Freely soluble in water, very slightly soluble in methanol, practically insoluble in ethanol (96 per cent) and in methylene chloride.
IDENTIFICATION
A. Infrared absorption spectrophotometry (2.2.24).
Comparison: iopamidol CRS.
B. Loss on drying (see Tests).
C. Specific optical rotation (see Tests).
TESTS
Appearance of solution
The solution is clear (2.2.1) and colourless (2.2.2, Method II).
Dissolve 1 g in water R and dilute to 50 mL with the same solvent.
Acidity or alkalinity
Dissolve 10.0 g in carbon dioxide-free water R and dilute to 100 mL with the same solvent. Not more than 0.75 mL of 0.01 M hydrochloric acid or 1.4 mL of 0.01 M sodium hydroxide is required to adjust to pH 7.0 (2.2.3).
Specific optical rotation (2.2.7)
-4.6 to -5.2 (dried substance), determined at 436 nm.
Dissolve 10.0 g, with heating if necessary, in water R and dilute to 25.0 mL with the same solvent.
Related substances
Liquid chromatography (2.2.29).
Test solution: Dissolve 0.50 g of the substance to be examined in water R and dilute to 50.0 mL with the same solvent.
Reference solution (a): Dissolve 5.0 mg of iopamidol impurity H CRS in water R and dilute to 100.0 mL with the same solvent.
Reference solution (b): Dilute 2.0 mL of the test solution to 20.0 mL with water R. Dilute 1.0 mL of this solution to 50.0 mL with water R.
Reference solution (c): Add 0.1 mL of the test solution to 20 mL of reference solution (a) and dilute to 50 mL with water R.
Column 2 columns coupled in series,
— size: l = 0.25 m, Ø = 4.6 mm,
— stationary phase: phenylsilyl silica gel for chromatography R (5 μm),
— temperature: 60 °C.
Mobile phase:
— mobile phase A: water R,
— mobile phase B: acetonitrile R, water R (50:50 V/V),
| Time
(min) |
Mobile phase A
(per cent V/V) |
Mobile phase B
(per cent V/V) |
| 0 – 18 | 100 | 0 |
| 18 – 40 | 100 – 62 | 0 – 38 |
| 40 – 45 | 62 – 50 | 38 – 50 |
| 45 – 50 | 50 – 100 | 50 – 0 |
| 50 – 60 | 100 | 0 |
Flow rate: 2.0 mL/min.
Detection: Spectrophotometer at 240 nm.
Injection: 20 μL.
Relative retention: With reference to iopamidol (retention time = about 14.6 min): impurity D = about 0.1; impurity B = about 0.6; impurities I and H = about 0.9; impurity G = about 1.1; impurity K = about 1.2; impurity C = about 1.3; impurity J = about 1.5; impurity A = about 1.8; impurity E = about 2.2; impurity F = about 2.3.
System suitability: Reference solution (c):
— resolution: minimum 2.0 between the peaks due to impurity H and iopamidol.
Limits:
— sum of impurities H and I: not more than the area of the principal peak in the chromatogram obtained with reference solution (a) (0.5 per cent),
— impurities A, B, C, D, E, F, G, J, K: for each impurity, not more than 0.5 times the area of the principal peak in the chromatogram obtained with reference solution (b) (0.1 per cent),
— any other impurity: for each impurity, not more than 0.5 times the area of the principal peak in the chromatogram obtained with reference solution (b) (0.1 per cent),
— sum of impurities other than H and I: not more than the area of the principal peak in the chromatogram obtained with reference solution (b) (0.2 per cent),
— disregard limit: 0.05 times the area of the principal peak in the chromatogram obtained with reference solution (b)(0.01 per cent).
Free aromatic amines
Maximum 200 ppm.
Keep the solutions and reagents in iced water, protected from bright light.
Test solution: In a 25 mL volumetric flask, dissolve 0.500 g of the substance to be examined in 20.0 mL of water R.
Reference solution: In a 25 mL volumetric flask, mix 4.0 mL of a 25.0 mg/L solution of iopamidol impurity A CRS with 16.0 mL of water R.
Blank solution: Place 20.0 mL of water R in a 25 mL volumetric flask.
Place the flasks in iced water, protected from light, for 5 min. Add 1.0 mL of hydrochloric acid R to each flask, mix and allow to stand for 5 min. Add 1.0 mL of a 20 g/L solution of sodium nitrite R prepared immediately before use, mix and allow to stand for 5 min. Add 1.0 mL of a 120 g/L solution of ammonium sulfamate R, swirl gently until gas liberation has ceased, and allow to stand for 5 min. (CAUTION: considerable pressure is produced). Add 1.0 mL of a freshly prepared 1 g/L solution of naphthylethylenediamine dihydrochloride R and mix. Remove the flasks from the iced water and allow to stand for 10 min. Dilute to 25.0 mL with water R and mix. Measure immediately the absorbance (2.2.25) at 500 nm of the solutions obtained from the test solution and the reference solution using, as the compensation liquid, the solution obtained from the blank solution.
The absorbance of the test solution is not greater than that of the reference solution.
Free iodine
Maximum 10 ppm.
Dissolve 2.0 g in 25 mL of water R in a ground-glass stoppered centrifuge tube. Add 5 mL of toluene R and 5 mL of dilute sulfuric acid R. Shake and centrifuge. Any red colour of the upper layer is not more intense than that of the upper phase obtained in the same way from 22 mL of water R, 2 mL of iodide standard solution (10 ppm I) R, 5 mL of dilute sulfuric acid R, 1 mL of strong hydrogen peroxide solution R and 5 mL of toluene R.
Iodide
Maximum 10 ppm.
Dissolve 6.000 g in water R and dilute to 20 mL with the same solvent. Add 2.0 mL of 0.001 M potassium iodide. Carry out a potentiometric titration (2.2.20) with 0.001 M silver nitrate using a silver indicator electrode and an appropriate reference electrode. Subtract the volume of titrant corresponding to the 2.0 mL of 0.001 M potassium iodide, determined by titrating a blank to which is added 2.0 mL of 0.001 M potassium iodide and use the residual value to calculate the iodide content.
1 mL of 0.001 M silver nitrate is equivalent to 126.9 μg of iodide.
Loss on drying (2.2.32)
Maximum 0.5 per cent, determined on 1.000 g by drying in an oven at 105 °C.
Sulfated ash (2.4.14)
Maximum 0.1 per cent, determined on 1.0 g.
Bacterial endotoxins (2.6.14)
Less than 1.4 IU/g, if intended for use in the manufacture of parenteral preparations without a further appropriate procedure for the removal of bacterial endotoxins.
ASSAY
To 0.300 g in a 250 mL round-bottomed flask add 5 mL of strong sodium hydroxide solution R, 20 mL of water R, 1 g of zinc powder R and a few glass beads. Boil under a reflux condenser for 30 min. Allow to cool and rinse the condenser with 20 mL of water R, adding the rinsings to the flask. Filter through a sintered-glass filter (2.1.2) and wash the filter with several quantities of water R. Collect the filtrate and washings. Add 5 mL of glacial acetic acid R and titrate immediately with 0.1 M silver nitrate. Determine the end-point potentiometrically (2.2.20) using a suitable electrode system such as
silver-silver chloride.
l mL of 0.1 M silver nitrate is equivalent to 25.90 mg of C17H22I3N3O8.
STORAGE
Protected from light. If the substance is sterile, store in a sterile, airtight, tamper-evident container.
IMPURITIES
Specified impurities A, B, C, D, E, F, G, H, I, J, K.
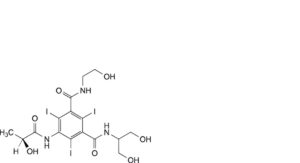
A. 5-amino-N,N’-bis[2-hydroxy-1-(hydroxymethyl)ethyl]-2,4,6-triiodobenzene-1,3-dicarboxamide,
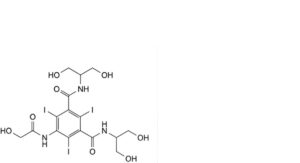
B. 5-[(hydroxyacetyl)amino]-N,N’-bis[2-hydroxy-1-(hydroxymethyl)ethyl]-2,4,6-triiodobenzene-1,3-dicarboxamide,
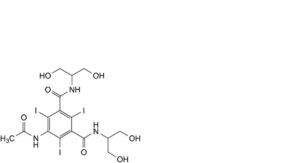
C. 5-(acetylamino)-N,N’-bis[2-hydroxy-1-(hydroxymethyl)ethyl]-2,4,6-triiodobenzene-1,3-dicarboxamide,
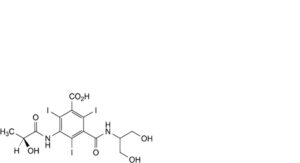
D. 3-[[2-hydroxy-1-(hydroxymethyl)ethyl]carbamoyl]-5-[[(2S)-2-hydroxypropanoyl]amino]-2,4,6-triiodobenzoic acid,
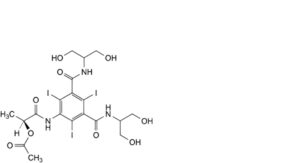
E. (1S)-2-[[3,5-bis[[2-hydroxy-1-(hydroxymethyl)ethyl]carbamoyl]-2,4,6-triiodophenyl]amino]-1-methyl-2-oxoethyl acetate,
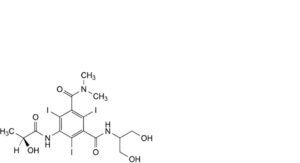
F. N’-[2-hydroxy-1-(hydroxymethyl)ethyl]-5-[[(2S)-2-hydroxypropanoyl]amino]-2,4,6-triiodo-N,N-dimethylbenzene-1,3-dicarboxamide,
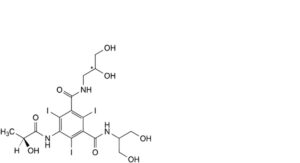
G. N-(2,3-dihydroxypropyl)-N’-[2-hydroxy-1-(hydroxymethyl)ethyl]-5-[[(2S)-2-hydroxypropanoyl]amino]-2,4,6-triiodobenzene-1,3-dicarboxamide,
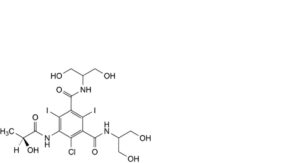
H. 4-chloro-N,N’-bis[2-hydroxy-1-(hydroxymethyl)ethyl]-5-[[(2S)-2-hydroxypropanoyl]amino]-2,6-diiodobenzene-1,3-dicarboxamide,
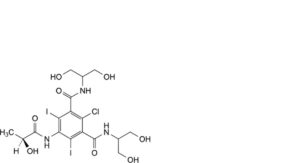
I. 2-chloro-N,N’-bis[2-hydroxy-1-(hydroxymethyl)ethyl]-5-[[(2S)-2-hydroxypropanoyl]amino]-4,6-diiodobenzene-1,3-dicarboxamide,
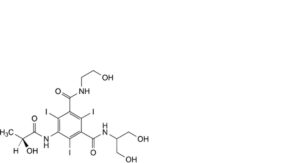
J. N-(2-hydroxyethyl)-N’-[2-hydroxy-1-(hydroxymethyl)ethyl]-5-[[(2S)-2-hydroxypropanoyl]amino]-2,4,6-triiodobenzene-1,3-dicarboxamide,
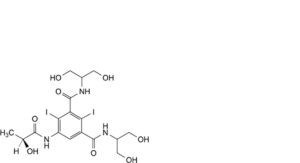
K. N,N’-bis[2-hydroxy-1-(hydroxymethyl)ethyl]-5-[[(2S)-2-hydroxypropanoyl]amino]-2,4-diiodobenzene-1,3-dicarboxamide.