(Ph. Eur. monograph 1114)
C19H26I3N3O9 821 66108-95-0
Action and use
Iodinated contrast medium.
DEFINITION
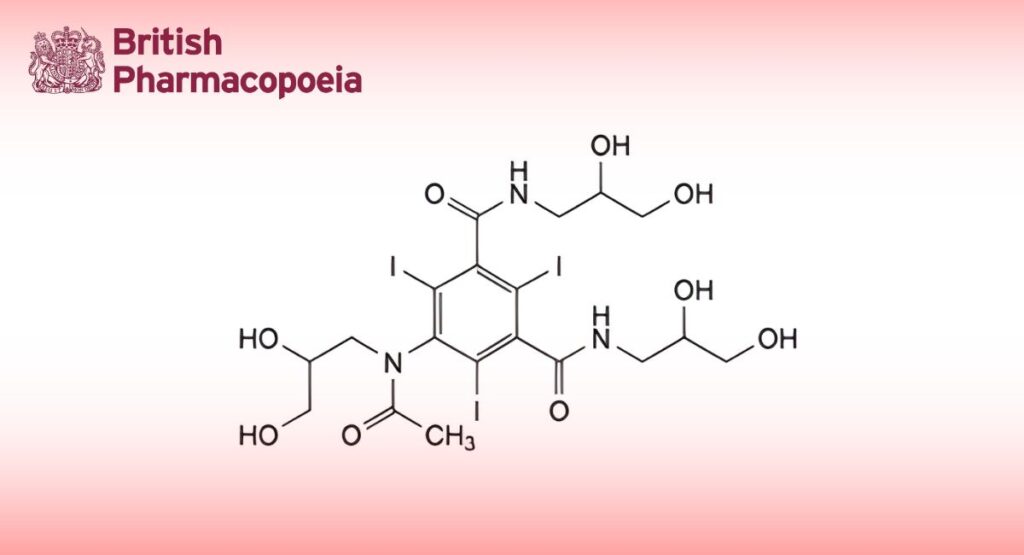
5-[Acetyl(2,3-dihydroxypropyl)amino]-N,N′-bis(2,3-dihydroxypropyl)-2,4,6-triiodobenzene-1,3-dicarboxamide.
The substance is a mixture of diastereoisomers and atropisomers.
Content
98.0 per cent to 101.0 per cent (anhydrous substance).
CHARACTERS
Appearance
White or greyish-white, hygroscopic powder.
Solubility
Very soluble in water, freely soluble in methanol, practically insoluble in methylene chloride.
IDENTIFICATION
A. Infrared absorption spectrophotometry (2.2.24).
Comparison: iohexol CRS.
B. Examine the chromatograms obtained in test A for related substances.
Results: The principal peaks in the chromatogram obtained with reference solution (b) are similar in retention time and size to the peaks due to iohexol in the chromatogram obtained with reference solution (a).
TESTS
Solution S
Dissolve 5.0 g in water R and dilute to 50.0 mL with the same solvent.
Appearance of solution
Solution S is clear (2.2.1) and not more intensely coloured than reference solution Y7 (2.2.2, Method II).
Related substances
A. Liquid chromatography (2.2.29).
NOTE: iohexol gives rise to 2 non-resolved peaks in the chromatogram due to endo-exo isomerism. In addition, a small peak (also due to iohexol) usually appears at the leading edge of the 1 principal peak. This small peak has a retention
time about 1.2 min less than the 1 principal peak.
Test solution: Dissolve 0.150 g of the substance to be examined in water R and dilute to 100.0 mL with the same solvent.
Reference solution (a): Dissolve 15.0 mg of iohexol CRS and 15.0 mg of iohexol impurity A CRS in a mixture of 0.05- 0.1 mL of dilute sodium hydroxide solution R and 10 mL of water R and dilute to 100.0 mL with water R. Dilute 1.0 mL of
this solution to 10.0 mL with water R.
Reference solution (b): Dilute 1.0 mL of the test solution to 100.0 mL with water R.
Reference solution (c): Dissolve 5.0 mg of iohexol for peak identification CRS (containing impurities B, C, D and E) in water R and dilute to 5.0 mL with the same solvent.
Blank solution: water R.
Column:
— size: l = 0.25 m, Ø = 4.6 mm;
— stationary phase: octadecylsilyl silica gel for chromatography R (5 μm).
Mobile phase:
— mobile phase A: water R;
— mobile phase B: acetonitrile R;
| Time (min) |
Mobile phase A (per cent V/V) |
Mobile phase B (per cent V/V) |
| 0 – 60 | 99 → 87 | 1 → 13 |
Flow rate: 1 mL/min.
Detection: Spectrophotometer at 254 nm.
Injection: 10 μL.
Retention time: Impurities A and H = about 17 min; iohexol (peaks corresponding to endo-exo isomerism) = about 20 min.
System suitability: Reference solution (a):
— resolution: minimum 5.0 between the peak due to impurity A and the 2 and greater peak due to iohexol.
Limits:
— sum of impurities B, C, D and E (relative retention with reference to the 2 and greater peak due to iohexol between 1.1 and 1.4): not more than 0.6 times the total area of the principal peaks in the chromatogram obtained with reference solution (b) (0.6 per cent); use the chromatogram obtained with reference solution (c) to identify the corresponding peaks;
— sum of impurities A and H: not more than 0.5 times the total area of the principal peaks in the chromatogram obtained with reference solution (b) (0.5 per cent);
— unspecified impurities: for each impurity, not more than 0.1 times the total area of the principal peaks in the chromatogram obtained with reference solution (b) (0.10 per cent);
— total: not more than 1.5 times the total area of the principal peaks in the chromatogram obtained with reference solution (b) (1.5 per cent);
— disregard limit: 0.03 times the total area of the principal peaks in the chromatogram obtained with reference solution (b) (0.03 per cent).
The thresholds indicated under Related substances (Table 2034.-1) in the general monograph Substances for pharmaceutical use (2034) do not apply.
B. Thin-layer chromatography (2.2.27).
Test solution: Dissolve 1.0 g of the substance to be examined in water R and dilute to 10.0 mL with the same solvent.
Reference solution (a): Dissolve 50 mg of iohexol impurity J CRS and 50 mg of iohexol CRS in water R and dilute to 10.0 mL with the same solvent.
Reference solution (b): Dilute 1.0 mL of the test solution to 10.0 mL with water R. Dilute 1.0 mL of this solution to 50.0 mL with water R.
Plate: TLC silica gel F254 plate R.
Pretreatment: Wash the plate with the mobile phase, dry at room temperature for 30 min, then at 90 °C for 1 h.
Mobile phase: concentrated ammonia R, methanol R, 2-propanol R, acetone R (16:16:28:40 V/V/V/V).
Application: 10 μL.
Development: Over 1/2 of the plate.
Drying: In air.
Detection: Examine in ultraviolet light at 254 nm.
System suitability: Reference solution (a):
— the chromatogram shows 2 clearly separated spots.
Limits:
— any impurity: any spot in the chromatogram obtained with the test solution, apart from the principal spot, is not more intense than the spot in the chromatogram obtained with reference solution (b) (0.2 per cent).
The thresholds indicated under Related substances (Table 2034.-1) in the general monograph Substances for pharmaceutical use (2034) do not apply.
3-Chloropropane-1,2-diol
Gas chromatography (2.2.28).
Test solution: Dissolve 1.0 g of the substance to be examined in 1.0 mL of water R. Shake with 4 quantities, each of 2 mL, of methyl acetate R. Dry the combined upper layers over anhydrous sodium sulfate R. Filter and concentrate to about 0.7 mL using a warm water-bath at 60 °C and a stream of nitrogen and dilute to 1.0 mL with methyl acetate R.
Reference solution: Dissolve 0.25 g of 3-chloropropane-1,2-diol R in 100.0 mL of methyl acetate R. Dilute 1.0 mL of this solution to 100.0 mL with methyl acetate R.
Column:
— material: fused silica;
— size: l = 25 m, Ø = 0.33 mm;
— stationary phase: phenyl(50)methyl(50)polysiloxane R (film thickness 1 μm).
Carrier gas: helium for chromatography R.
Flow rate: 1 mL/min.
Temperature:
| Time (min) |
Temperature (°C) |
|
| Column | 0 – 2 | 80 |
| 2 – 8 | 80 → 170 | |
| 8 – 10 | 170 | |
| Injection port | 230 | |
| Detector | 250 |
Detection: Flame ionisation.
Injection: 2 μL (splitless for 30 s).
System suitability: Reference solution:
— retention time: 3-chloropropane-1,2-diol = about 8 min.
Limit:
— 3-chloropropane-1,2-diol: not more than the area of the principal peak in the chromatogram obtained with the reference solution (25 ppm).
Free aromatic amine
Maximum 500 ppm.
Test solution: Transfer 0.200 g of the substance to be examined to a 25 mL volumetric flask and dissolve in 15.0 mL of water R.
Reference solution: Dissolve 5.0 mg of iohexol impurity J CRS in water R and dilute to 5.0 mL with water R. Dilute 1.0 mL of the solution to 100.0 mL with water R. Mix 10.0 mL of this solution with 5.0 mL of water R in a 25 mL volumetric flask.
Blank solution: Transfer 15.0 mL of water R to a 25 mL volumetric flask.
In conducting the following steps, keep the flasks in iced water and protected as much as possible from light until all of the reagents have been added.
Place the 3 flasks containing respectively the test solution, the reference solution and the blank solution in iced water, protected from light, for 5 min. Add 1.5 mL of hydrochloric acid R1 and mix by swirling. Add 1.0 mL of a 20 g/L solution of sodium nitrite R, mix and allow to stand for 4 min. Add 1.0 mL of a 40 g/L solution of sulfamic acid R, swirl gently until gas liberation has ceased and allow to stand for 1 min. (CAUTION: considerable pressure is produced). Add 1.0 mL of a freshly prepared 3 g/L solution of naphthylethylenediamine dihydrochloride R in a mixture of 30 volumes of water R and 70 volumes of propylene glycol R and mix. Remove the flasks from the iced water, dilute to 25.0 mL with water R, mix and allow to stand for 5 min. Simultaneously determine the absorbance (2.2.25) at 495 nm of the solutions obtained from the test solution and the reference solution in 5 cm cells, using the blank as the compensation liquid. The absorbance of the test solution is not greater than that of the reference solution.
Iodide
Maximum 10 ppm.
Dissolve 6.000 g in water R and dilute to 20 mL with the same solvent. Add 2.0 mL of 0.001 M potassium iodide. Titrate with 0.001 M silver nitrate. Determine the end-point potentiometrically (2.2.20), using a silver indicator electrode and an appropriate reference electrode. Subtract the volume of titrant corresponding to the 2.0 mL of 0.001 M potassium iodide, determined by titrating a blank to which is added 2.0 mL of 0.001 M potassium iodide and use the residual value to calculate the iodide content.
1 mL of 0.001 M silver nitrate is equivalent to 126.9 μg of I .
Ionic compounds (2.2.38)
Maximum 0.01 per cent m/m calculated as sodium chloride.
Rinse all glassware with distilled water R 5 times before use.
Test solution: Dissolve 1.0 g of the substance to be examined in water R and dilute to 50.0 mL with the same solvent.
Reference solution: Dissolve 20.0 mg of sodium chloride R in water R and dilute to 100.0 mL with the same solvent. Dilute 1.0 mL of this solution to 100.0 mL with water R.
Measure the conductivity of the test solution and the reference solution using a suitable conductivity meter. The conductivity of the test solution is not greater than that of the reference solution.
Water (2.5.12)
Maximum 4.0 per cent, determined on 1.00 g.
ASSAY
To 0.500 g in a 125 mL round-bottomed flask add 25 mL of a 50 g/L solution of sodium hydroxide R, 0.5 g of zinc powder R and a few glass beads. Boil under a reflux condenser for 30 min. Allow to cool and rinse the condenser with 20 mL of
water R, adding the rinsings to the flask. Filter through a sintered-glass filter (2.1.2) and wash the filter with several quantities of water R. Collect the filtrate and washings. Add 5 mL of glacial acetic acid R and titrate immediately with 0.1 M
silver nitrate. Determine the end-point potentiometrically (2.2.20).
1 mL of 0.1 M silver nitrate is equivalent to 27.37 mg of C19H26I3N3O9.
STORAGE
In an airtight container, protected from light and moisture.
IMPURITIES
Specified impurities A, B, C, D, E, H.
Other detectable impurities (the following substances would, if present at a sufficient level, be detected by one or other of the tests in the monograph. They are limited by the general acceptance criterion for other/unspecified impurities. It is
therefore not necessary to identify these impurities for demonstration of compliance. See also 5.10. Control of impurities in substances for pharmaceutical use) F, G, I, J, K, L, M, N, O, P, Q.
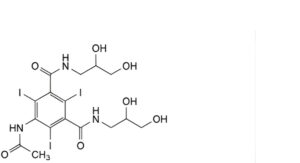
A. 5-(acetylamino)-N,N′-bis(2,3-dihydroxypropyl)-2,4,6-triiodobenzene-1,3-dicarboxamide,
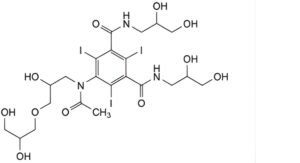
B. 5-[acetyl[3-(2,3-dihydroxypropoxy)-2-hydroxypropyl]amino]-N,N′-bis(2,3-dihydroxypropyl)-2,4,6-triiodobenzene-1,3- dicarboxamide,
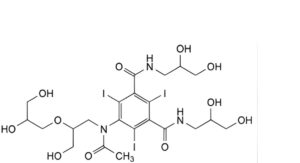
C. 5-[acetyl[2-(2,3-dihydroxypropoxy)-3-hydroxypropyl]amino]-N,N′-bis(2,3-dihydroxypropyl)-2,4,6-triiodobenzene-1,3- dicarboxamide,
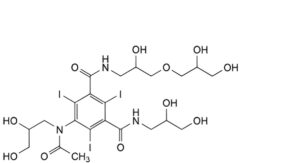
D. 5-[acetyl(2,3-dihydroxypropyl)amino]-N-[3-(2,3-dihydroxypropoxy)-2-hydroxypropyl]-N′-(2,3-dihydroxypropyl)-2,4,6- triiodobenzene-1,3-dicarboxamide,
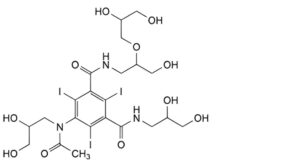
E. 5-[acetyl(2,3-dihydroxypropyl)amino]-N-[2-(2,3-dihydroxypropoxy)-3-hydroxypropyl]-N′-(2,3-dihydroxypropyl)-2,4,6- triiodobenzene-1,3-dicarboxamide,
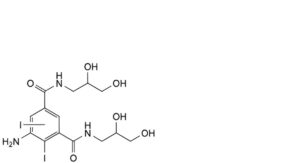
F. 5-amino-N,N′-bis(2,3-dihydroxypropyl)diiodobenzene-1,3-dicarboxamide,
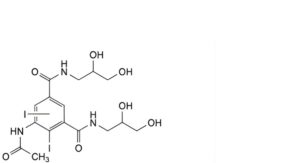
G. 5-(acetylamino)-N,N′-bis(2,3-dihydroxypropyl)diiodobenzene-1,3-dicarboxamide,
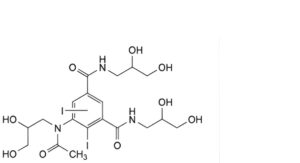
H. 5-[acetyl(2,3-dihydroxypropyl)amino]-N,N′-bis(2,3-dihydroxypropyl)diiodobenzene-1,3-dicarboxamide,
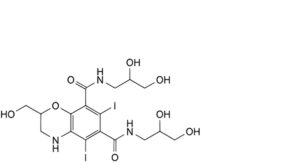
I. N,N′-bis(2,3-dihydroxypropyl)-2-(hydroxymethyl)-5,7-diiodo-3,4-dihydro-2H-1,4-benzoxazine-6,8-dicarboxamide,
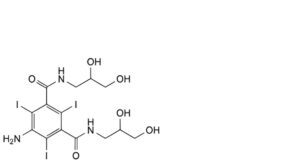
J. 5-amino-N,N′-bis(2,3-dihydroxypropyl)-2,4,6-triiodobenzene-1,3-dicarboxamide,

K. 5-amino-2,4,6-triiodobenzene-1,3-dicarboxylic acid,
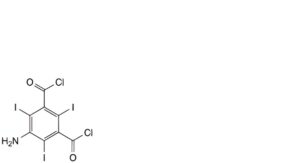
L. 3,5-bis(chlorocarbonyl)-2,4,6-triiodobenzenamine,
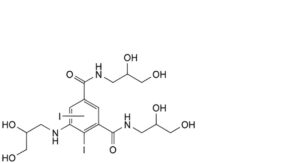
M. N,N′-bis(2,3-dihydroxypropyl)-5-[(2,3-dihydroxypropyl)amino]diiodobenzene-1,3-dicarboxamide,
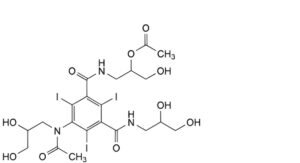
N. 5-[acetyl(2,3-dihydroxypropyl)amino]-N-[2-(acetyloxy)-3-hydroxypropyl]-N′-(2,3-dihydroxypropyl)-2,4,6-triiodobenzene- 1,3-dicarboxamide,
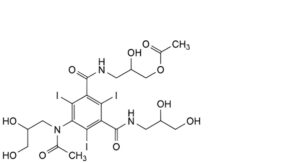
O. 5-[acetyl(2,3-dihydroxypropyl)amino]-N-[3-(acetyloxy)-2-hydroxypropyl]-N′-(2,3-dihydroxypropyl)-2,4,6-triiodobenzene- 1,3-dicarboxamide,
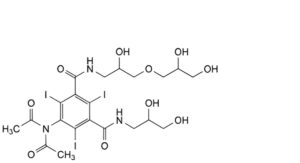
P. 5-(diacetylamino)-N-[3-(2,3-dihydroxypropoxy)-2-hydroxypropyl]-N′-(2,3-dihydroxypropyl)-2,4,6-triiodobenzene-1,3- dicarboxamide,
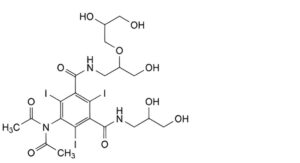
Q. 5-(diacetylamino)-N-[2-(2,3-dihydroxypropoxy)-3-hydroxypropyl]-N′-(2,3-dihydroxypropyl)-2,4,6-triiodobenzene-1,3- dicarboxamide.