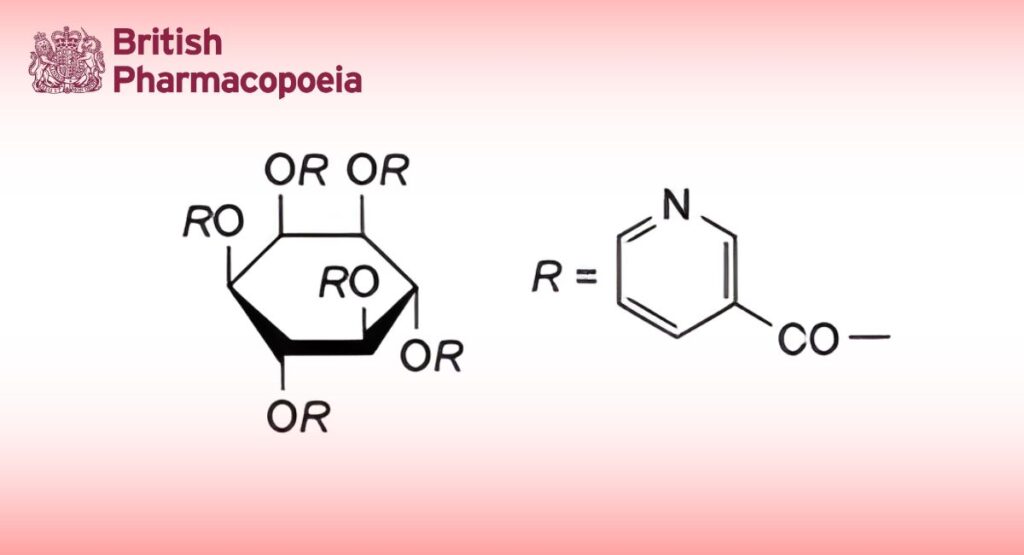
C42H30N6O12 810.7 6556-11-2
Action and use
Vasodilator.
Preparation
Inositol Nicotinate Tablets
DEFINITION
Inositol Nicotinate is myo-inositol hexanicotinate. It contains not less than 98.0% and not more than 101.0% of C42H30N6O12, calculated with reference to the dried substance.
CHARACTERISTICS
A white or almost white powder.
Practically insoluble in water; practically insoluble in acetone, in ethanol (96%) and in ether. It dissolves in dilute mineral acids.
IDENTIFICATION
The infrared absorption spectrum, Appendix II A, is concordant with the reference spectrum of inositol nicotinate (RS 190).
TESTS
Clarity and colour of solution
A 5.0% w/v solution in 0.5M sulfuric acid is clear, Appendix IV A, and not more intensely coloured than reference solution BY6, Appendix IV B, Method II.
Chloride
Dissolve 0.14 g in a sufficient quantity of 2M nitric acid and dilute to 16 mL with water. The resulting solution complies with the limit test for chlorides, Appendix VII, beginning at the words ‘pour the mixture as a single addition…’ (350 ppm).
Free nicotinic acid
To 1 g add 75 mL of water, shake for 15 minutes and titrate with 0.02M sodium hydroxide VS using phenolphthalein solution R1 as indicator. Not more than 0.8 mL of 0.02M sodium hydroxide VS is required to produce the first pink colour.
Related substances
Carry out the method for thin-layer chromatography, Appendix III A, using a plate 200 mm × 200 mm in size and silica gel GF254 as the coating substance. For the first development use a mixture of 90 volumes of chloroform and 10 volumes of methanol as the mobile phase. Apply to the bottom right-hand corner of the plate 5 μL of solution (1) containing 5.0% w/v of the substance being examined in a mixture of 9 volumes of chloroform and 1 volume of methanol and develop over a path of 12 cm. After removal of the plate, allow it to dry in air and turn the plate through 90° in a clockwise direction. Apply separately to the bottom right-hand corner of the plate, and to the right of the solvent front, 5 μL of each of two solutions of the substance being examined in a mixture of 9 volumes of chloroform and 1 volume of methanol containing (2) 0.075% w/v and (3) 0.050% w/v. For the second development use a mixture of 50 volumes of ethyl acetate and 5 volumes each of glacial acetic acid, ethanol (96%) and water as the mobile phase. After removal of the plate, allow it to dry in air and examine under ultraviolet light (254 nm). In the chromatogram obtained with solution (1) any secondary spot is not more intense than the spot in the chromatogram obtained with solution (2) (1.5%) and not more than one such spot is more intense than the spot in the chromatogram obtained with solution (3) (1%).
Acetone
Prepare a 0.020% v/v solution of butan-2-one (internal standard) in dimethylformamide (solution A). Carry out the method for gas chromatography, Appendix III B, using the following solutions. Solution (1) contains 0.020% v/v of acetone in solution A. For solution (2) add 5 mL of dimethylformamide to 0.20 g of the substance being examined contained in a suitable vessel, stopper securely, suspend in a water bath until solution is complete and allow to cool. Prepare solution (3) in the same manner as solution (2) but using 5 mL of solution A in place of the dimethylformamide.
The chromatographic procedure may be carried out using a glass column (1.5 m × 4 mm) packed with acid-washed, silanised diatomaceous support coated with 10% w/w of polyethylene glycol 1000 and maintained at 60°.
In the chromatogram obtained with solution (3) the ratio of the area of any peak corresponding to acetone to the area of the peak due to the internal standard is not greater than the corresponding ratio in the chromatogram obtained with solution (1).
Loss on drying
When dried to constant weight at 105°, loses not more than 0.5% of its weight. Use 1 g.
Sulfated ash
Not more than 0.1%, Appendix IX A.
ASSAY
Carry out Method I for non-aqueous titration, Appendix VIII A, using 0.2 g and 1-naphtholbenzein solution as indicator.
Each mL of 0.1M perchloric acid VS is equivalent to 13.51 mg of C42H30N6O12.