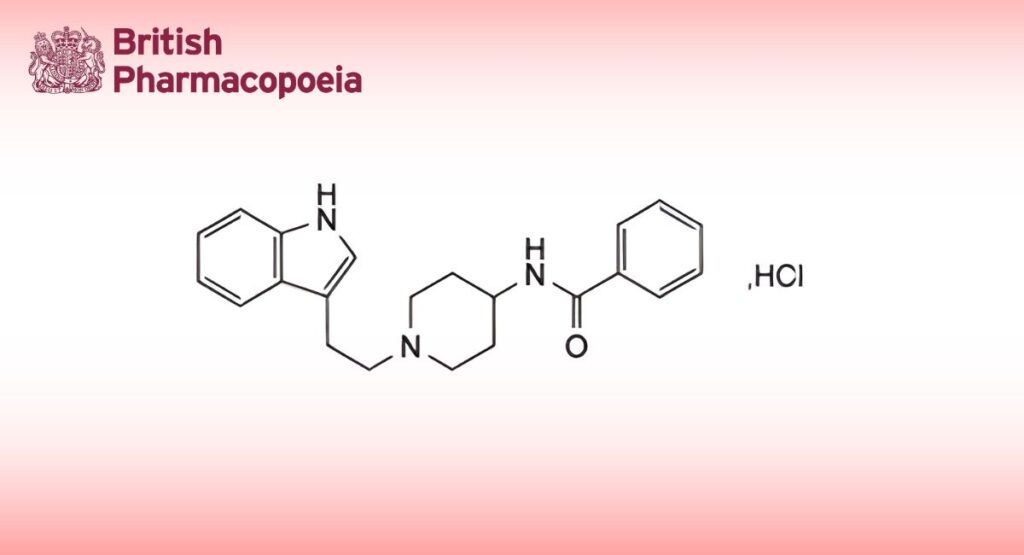
C22H25N3O,HCl 383.9 33124-53-7
Action and use
Alpha1-adrenoceptor antagonist.
Preparation
Indoramin Tablets
DEFINITION
Indoramin Hydrochloride is N-1-[2-(indol-3-yl)ethyl]-4-piperidylbenzamide hydrochloride. It contains not less than 98.5% and not more than 100.5% of C22H25N3O,HCl, calculated with reference to the dried substance.
CHARACTERISTICS
A white or almost white powder. It exhibits polymorphism.
Slightly soluble in water; sparingly soluble in ethanol (96%); soluble in methanol; very slightly soluble in ether.
IDENTIFICATION
A. The light absorption, Appendix II B, in the range 230 to 350 nm of a 0.0045% w/v solution in ethanol (96%) exhibits three maxima, at 273, 280 and 290 nm. The absorbances at the maxima are about 0.76, 0.77 and 0.64, respectively.
B. Dissolve 50 mg in 30 mL of water, make the solution alkaline by the addition of 5M ammonia and shake with 50 mL of dichloromethane. Dry the dichloromethane layer with anhydrous sodium sulfate, filter and evaporate the filtrate to dryness using a rotary evaporator. The infrared absorption spectrum of the residue, Appendix II A, is concordant with the reference spectrum of indoramin (RS 188).
C. Yields reaction A characteristic of chlorides, Appendix VI.
TESTS
Acidity
pH of a 2% w/v suspension in water, 4.0 to 5.5, Appendix V L.
Related substances
Carry out the method for thin-layer chromatography, Appendix III A, using a silica gel F254 precoated plate (Merck silica gel 60 F254 plates are suitable)and a mixture of 1 volume of 18M ammonia, 20 volumes of absolute ethanol and 79 volumes of toluene as the mobile phase. Apply separately to the plate 10 μL of each of three solutions of the substance being examined in ethanol (96%) containing (1) 1.0% w/v, (2) 0.0050% w/v and (3) 0.0010% w/v. After removal of the plate, allow it to dry in a current of warm air and examine under ultraviolet light (254 nm). Any secondary spot in the chromatogram obtained with solution (1) is not more intense than the spot in the chromatogram obtained with solution (2) (0.5%) and not
more than one such spot is more intense than the spot in the chromatogram obtained with solution (3) (0.1%).
Loss on drying
When dried at 100° to 105° for 4 hours, loses not more than 0.5% of its weight. Use 1 g.
Sulfated ash
Not more than 0.1%, Appendix IX A.
ASSAY
Dissolve 0.2 g in 30 mL of anhydrous acetic acid, add 6 mL of acetic anhydride and 6 mL of mercury(II) acetate solution. Titrate with 0.1M perchloric acid VS determining the end point potentiometrically. Each mL of 0.1M perchloric acid VS is equivalent to 38.39 mg of C22H25N3O,HCl.
STORAGE
Indoramin Hydrochloride should be protected from light.
IMPURITIES
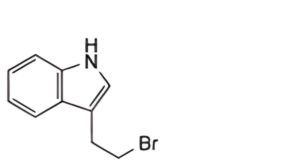
A. 3-(2-bromoethyl)indole
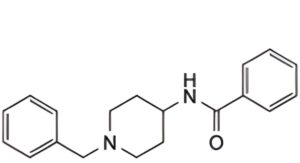
B. N-(1-benzyl-4-piperidyl)benzamide
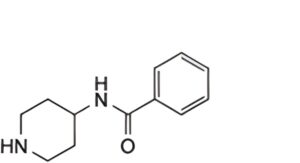
C. N-(4-piperidyl)benzamide