Edition: BP 2025 (Ph. Eur. 11.6 update)
Prolonged-release Indapamide Tablets
Indapamide Prolonged-release Tablets from different manufacturers, whilst complying with the requirements of the monograph, are not interchangeable unless otherwise justified and authorised.
Action and use
Thiazide-like diuretic.
DEFINITION
Indapamide Prolonged-release Tablets contain Indapamide. They are formulated so that the medicament is released over a period of several hours.
PRODUCTION
A suitable dissolution test is carried out to demonstrate the appropriate release of Indapamide. The dissolution profile reflects the in vivo performance which in turn is compatible with the dosage schedule recommended by the manufacturer.
A suitable test is carried out to demonstrate that the content of (2RS)-2-methyl-2,3-dihydro-1H-indol-1-amine (impurity C) is not more than 600 ppm.
The tablets comply with the requirements stated under Tablets and with the following requirements.
Content of indapamide, C16H16ClN3O3S
95.0 to 105.0% of the stated amount.
IDENTIFICATION
A. Carry out the method for thin-layer chromatography, Appendix III A, using the following solutions.
(1) Grind a quantity of the powdered tablets containing 50 mg of Indapamide with 10 mL of acetone, mix for 15 minutes, filter (Whatman 42 is suitable) and use the filtrate.
(2) 0.5% w/v of indapamide BPCRS in acetone.
CHROMATOGRAPHIC CONDITIONS
(a) Use as the coating TLC silica gel F254 (Merck silica gel 60 F254 plates are suitable).
(b) Use the mobile phase as described below.
(c) Apply 20 μL of each solution.
(d) Develop the plate to 12 cm.
(e) After removal of the plate, allow it to dry until the solvent has evaporated and examine under ultraviolet light (254 nm). Spray the plate with a solution prepared by mixing 10 volumes of potassium iodobismuthate solution and 20 volumes of glacial acetic acid and diluting to 100 volumes with water and examine again. Finally, spray the plate with a 5% w/v solution of sodium nitrite in a mixture of equal volumes of water and ethanol (96%) and examine again.
MOBILE PHASE
20 volumes of acetone and 80 volumes of toluene.
CONFIRMATION
By each method of visualisation, the principal spot in the chromatogram obtained with solution (1) is similar in position, colour and intensity to that in the chromatogram obtained with solution (2).
B. In the Assay, the retention time of the principal peak in the chromatogram obtained with solution (1) is similar to that of the principal peak in the chromatogram obtained with solution (2).
TESTS
Related substances
Carry out the method for liquid chromatography, Appendix III D, protected from light, using the following solutions prepared immediately before use.
(1) To a quantity of the powdered tablets containing 25 mg of Indapamide add 70 mL of ethanol (96%) and mix mechanically for 2 hours. Add sufficient ethanol (96%) to produce 100 mL, mix and centrifuge. Dilute the supernatant liquid with the mobile phase to produce a solution containing 0.005% w/v of Indapamide.
(2) Dilute 1 volume of solution (1) to 10 volumes with the mobile phase and further dilute 1 volume of this solution to 100 volumes with the mobile phase.
(3) Dilute 1 volume of a 0.00025% w/v solution of indapamide impurity B BPCRS in ethanol (96%) to 10 volumes with the mobile phase.
(4) Dilute 1 volume of a 0.00025% w/v solution of 4-chloro-3-sulfamoylbenzoic acid in ethanol (96%) to 10 volumes with the mobile phase.
(5) Mix 1 volume of solution (1), 1 volume of a 0.00025% w/v solution of indapamide impurity B BPCRS in ethanol (96%) and 1 volume of a 0.00025% w/v solution of 4-chloro-3-sulfamoylbenzoic acid in ethanol (96%) and add 7 volumes of the mobile phase.
CHROMATOGRAPHIC CONDITIONS
(a) Use a stainless steel column (15 cm × 4.6 mm) packed with octadecylsilyl silica gel for chromatography (5 μm) (Nucleosil C18 is suitable).
(b) Use isocratic elution and the mobile phase described below.
(c) Use a flow rate of 1.6 mL per minute.
(d) Use an ambient column temperature.
(e) Use a detection wavelength of 240 nm.
(f) Inject 20 μL of each solution.
MOBILE PHASE
6 volumes of a solution containing 5% w/v of sodium dodecyl sulfate and 3% v/v of glacial acetic acid, 10 volumes of triethylamine, 20 volumes of butan-2-ol, 310 volumes of acetonitrile and 690 volumes of water, the mixture being adjusted to pH 3.0 with orthophosphoric acid.
SYSTEM SUITABILITY
The test is not valid unless, in the chromatogram obtained with solution (5), the retention time of impurity B relative to indapamide is about 1.7 and the retention time of 4-chloro-3-sulfamoylbenzoic acid relative to indapamide is about 0.3.
LIMITS
In the chromatogram obtained with solution (1):
the area of any peak corresponding to impurity B is not greater than twice the area of the peak in the chromatogram obtained with solution (3) (1.0%);
the area of any peak corresponding to 4-chloro-3-sulfamoylbenzoic acid is not greater than the area of the peak in the chromatogram obtained with solution (4) (0.5%);
the area of any other secondary peak is not greater than 5 times the area of the peak in the chromatogram obtained with solution (2) (0.5%);
the sum of the impurities is not greater than 1.5%.
Disregard any peak with an area less than the area of the principal peak in the chromatogram obtained with solution (2) (0.1%).
Uniformity of Content
Tablets containing less than 2 mg of Indapamide comply with the requirement stated under Tablets using the following method of analysis. Carry out the method for liquid chromatography, Appendix III D, protected from light, using the following solutions.
(1) Add 5 mL of ethanol (96%) to one whole tablet and mix mechanically for 2 hours and centrifuge. Dilute 1 volume of the supernatant liquid to 5 volumes with the mobile phase.
(2) Dilute 1 volume of a 0.03% w/v solution of indapamide BPCRS in ethanol (96%) to 5 volumes with the mobile phase.
CHROMATOGRAPHIC CONDITIONS
The chromatographic conditions described under Related substances may be used.
DETERMINATION OF CONTENT
Calculate the content of C16H16ClN3O3S in each tablet using the declared content of C16H16ClN3O3S in indapamide BPCRS.
ASSAY
For tablets containing less than 2 mg and/or less than 2% w/w of Indapamide
Use the average of the individual results determined in the test for Uniformity of content.
For tablets containing 2 mg or more and 2% w/w or more of Indapamide
Weigh and powder 20 tablets. Carry out the method for liquid chromatography, Appendix III D, protected from light, using the following solutions.
(1) To a quantity of the powdered tablets containing 25 mg of Indapamide add 70 mL of ethanol (96%) and mix mechanically for 2 hours. Add sufficient ethanol (96%) to produce 100 mL, mix and centrifuge. Dilute the supernatant liquid with the mobile phase to produce a solution containing 0.005% w/v of Indapamide.
(2) Dilute 1 volume of a 0.025% w/v solution of indapamide BPCRS in ethanol (96%) to 5 volumes with the mobile phase.
CHROMATOGRAPHIC CONDITIONS
The chromatographic conditions described under Related substances may be used.
DETERMINATION OF CONTENT
Calculate the content of C16H16ClN3O3S in each tablet using the declared content of C16H16ClN3O3S in indapamide BPCRS.
STORAGE
Indapamide Prolonged-release Tablets should be protected from light.
IMPURITIES
The impurities limited by the requirements of this monograph include impurities B and C listed under Indapamide and the following:
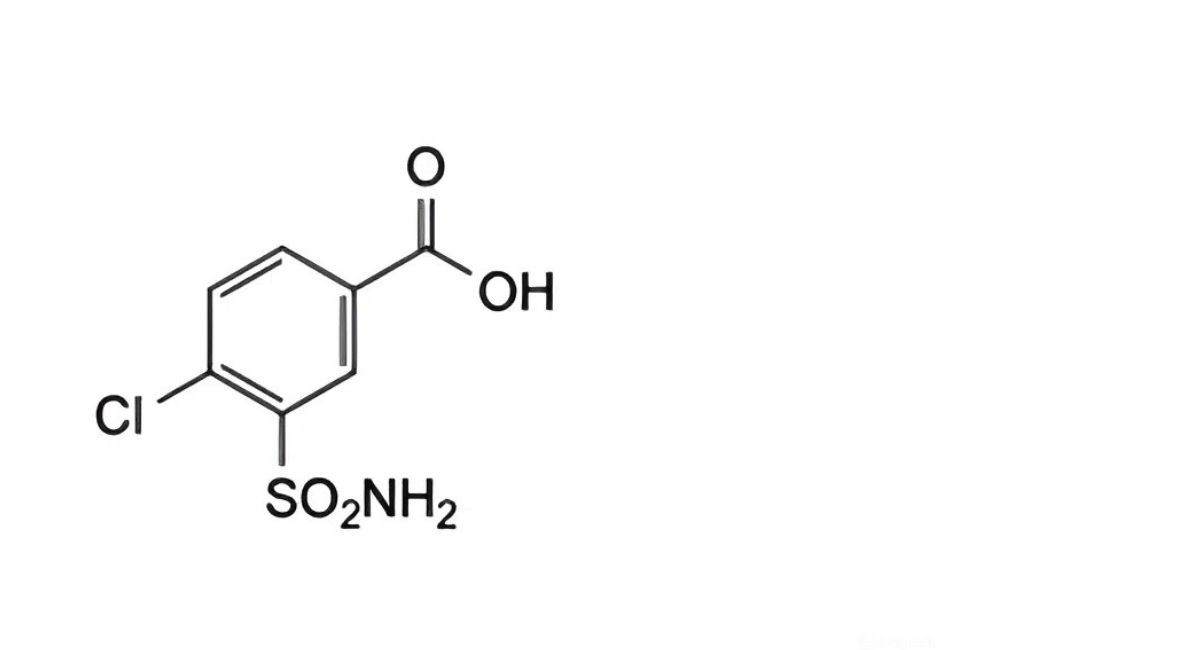
1. 4-chloro-3-sulfamoylbenzoic acid






