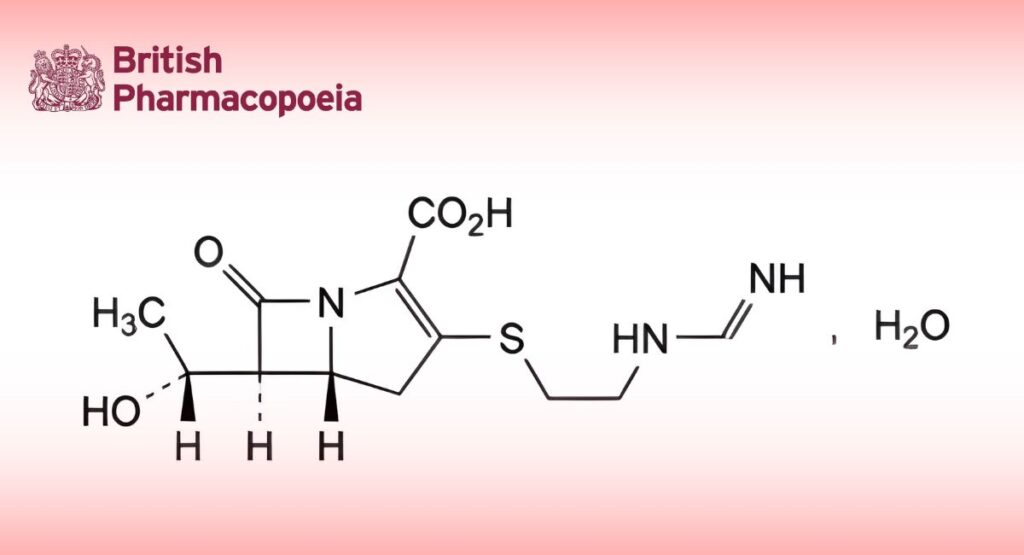
Imipenem
(Ph. Eur. monograph 1226)
C12H17N3O4S,H2O 317.4 74431-23-5
Action and use
Carbapenem antibacterial.
Preparation
Cilastatin and Imipenem for Infusion
DEFINITION
(5R,6S)-6-[(R)-1-Hydroxyethyl]-3-[[2-[(iminomethyl)amino]ethyl]sulfanyl]-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid monohydrate.
Semi-synthetic product derived from a fermentation product or obtained by any other means.
Content
98.0 per cent to 102.0 per cent (anhydrous substance).
CHARACTERS
Appearance
White or almost white or pale yellow powder, slightly hygroscopic.
Solubility
Slightly soluble in water and in methanol.
IDENTIFICATION
Infrared absorption spectrophotometry (2.2.24).
Comparison imipenem CRS.
TESTS
Appearance of solution
The solution is not more opalescent than reference suspension II (2.2.1) and not more intensely coloured than intensity 6 of the range of the reference solutions of the most appropriate colour (2.2.2, Method II).
Dissolve 0.500 g in phosphate buffer solution pH 7.0 R3 and dilute to 50 mL with the same solution.
pH (2.2.3)
4.5 to 7.5.
Dissolve 0.500 g in carbon dioxide-free water R and dilute to 100.0 mL with the same solvent.
Specific optical rotation (2.2.7)
+ 90 to + 95 (anhydrous substance), measured at 25 °C. Prepare the solutions immediately before use.
Dissolve 0.125 g in phosphate buffer solution pH 7.0 R3 and dilute to 25.0 mL with the same solution.
Related substances
Liquid chromatography (2.2.29). Prepare the solutions immediately before use.
Buffer solution A Dissolve 0.32 g of anhydrous sodium dihydrogen phosphate R and 1.04 g of anhydrous disodium hydrogen phosphate R in 900 mL of water for chromatography R. Adjust to pH 7.3 with dilute phosphoric acid R and dilute to 1000 mL with water for chromatography R.
Buffer solution B Dissolve 0.11 g of anhydrous disodium hydrogen phosphate R in 900 mL of water R.
Adjust to pH 6.8 with dilute phosphoric acid R and dilute to 1000 mL with water R.
Solvent mixture acetonitrile R, buffer solution B (0.7:99.3 V/V).
Test solution: Dissolve 25.0 mg of the substance to be examined in the solvent mixture and dilute to 25.0 mL with the solvent mixture.
Reference solution (a): Dissolve 25.0 mg of imipenem CRS in the solvent mixture and dilute to 25.0 mL with the solvent mixture.
Reference solution (b): Dilute 1.0 mL of the test solution to 100.0 mL with the solvent mixture.
Reference solution (c): Dissolve 5 mg of the substance to be examined in 8 mL of a mixture of 1 volume of dilute sulfuric acid R and 200 volumes of water R. After 5 min, add 10 mg of sodium carbonate R and dilute to 10.0 mL with water R.
Column:
— size: l = 0.15 m, Ø = 4.6 mm;
— stationary phase: end-capped octadecylsilyl silica gel for chromatography R (3 μm);
— temperature: 30 °C.
Mobile phase:
— mobile phase A: acetonitrile R1, buffer solution A (0.7:99.3 V/V);
— mobile phase B: acetonitrile R1, buffer solution A (25:75 V/V);
| Time (min) |
Mobile phase A (per cent V/V) |
Mobile phase B (per cent V/V) |
| 0 – 9 | 100 | 0 |
| 9 – 24 | 100 → 68 | 0 → 32 |
| 24 – 24.5 | 68 → 50 | 32 → 50 |
| 24.5 – 29 | 50 | 50 |
Flow rate 1.0 mL/min.
Detection Spectrophotometer at 210 nm.
Autosampler Set at 5 °C.
Injection 10 μL of the test solution and reference solutions (b) and (c).
Identification of impurities Use the chromatogram obtained with reference solution (c) to identify the peaks due to the epimers of impurity B.
Relative retention With reference to imipenem (retention time = about 8 min): epimer I of impurity B = about 0.33; epimer II of impurity B = about 0.35; impurity A = about 0.8.
System suitability Reference solution (c):
— peak-to-valley ratio: minimum 2.0, where Hp = height above the baseline of the peak due to epimer I of impurity B and Hv = height above the baseline of the lowest point of the curve separating this peak from the peak due to epimer II of impurity B.
Calculation of percentage contents:
— correction factor: multiply the peak area of impurity A by 2.4;
— for each impurity, use the concentration of imipenem monohydrate in reference solution (b).
Limits:
— impurity A: maximum 1.0 per cent;
— impurity B: for each epimer, maximum 0.3 per cent;
— unspecified impurities: for each impurity, maximum 0.10 per cent;
— total: maximum 1.5 per cent;
— reporting threshold: 0.05 per cent.
Water (2.5.12)
5.0 per cent to 8.0 per cent, determined on 0.100 g. Use an iodosulfurous reagent containing imidazole instead of pyridine and a clean container for each determination.
Sulfated ash (2.4.14)
Maximum 0.2 per cent, determined on 1.0 g.
Bacterial endotoxins (2.6.14)
Less than 0.17 IU/mg, if intended for use in the manufacture of parenteral preparations without a further appropriate procedure for removal of bacterial endotoxins.
ASSAY
Liquid chromatography (2.2.29) as described in the test for related substances with the following modification.
Injection Test solution and reference solution (a).
Calculate the percentage content of C12H17N3O4S taking into account the assigned content of imipenem CRS.
STORAGE
In an airtight container, at a temperature of 2 °C to 8 °C. If the substance is sterile, store in a sterile, airtight, tamper-evident container.
IMPURITIES
Specified impurities A, B.
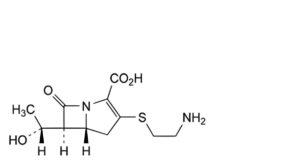
A. (5R,6S)-3-[(2-aminoethyl)sulfanyl]-6-[(R)-1-hydroxyethyl]-7-oxo-1-azabicyclo[3.2.0]hept-2-ene- 2-carboxylic acid(thienamycin),
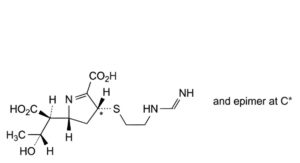
B. (2R,4RS)-2-[(1S,2R)-1-carboxy-2-hydroxypropyl]-4-[[2-[(iminomethyl)amino]ethyl]sulfanyl]-3,4-dihydro-2H-pyrrole-5-carboxylic acid (imipenemoic acid).