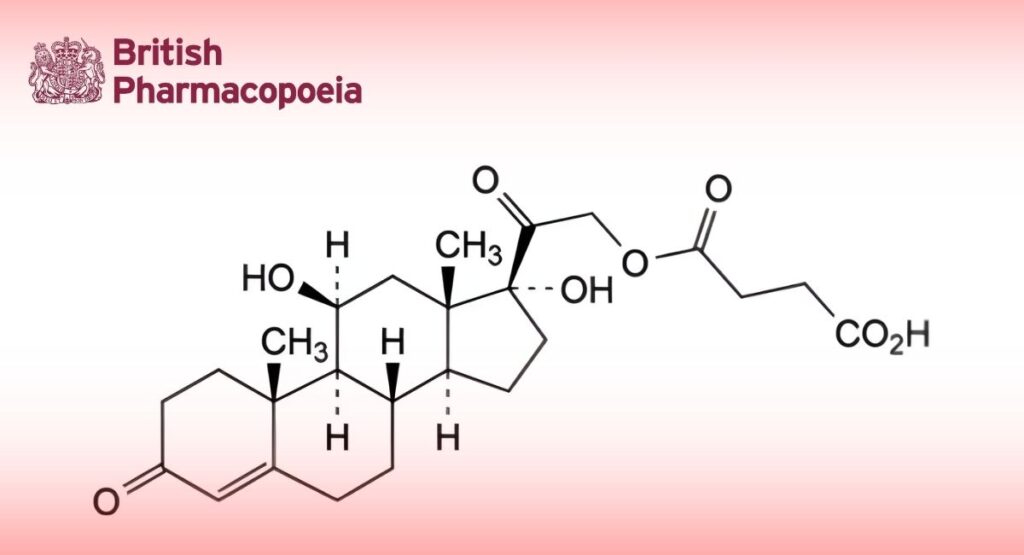
(Ph. Eur. monograph 0768)
C25H34O8 462.5 2203-97-6
Action and use
Corticosteroid.
Preparations
Hydrocortisone Sodium Succinate Injection
Hydrocortisone Oromucosal Tablets
DEFINITION
11β,17-Dihydroxy-3,20-dioxopregn-4-en-21-yl hydrogen butanedioate.
Content
97.0 per cent to 103.0 per cent (dried substance).
CHARACTERS
Appearance
White or almost white, hygroscopic powder.
Solubility
Practically insoluble in water, freely soluble in acetone and in anhydrous ethanol. It dissolves in dilute solutions of alkali carbonates and alkali hydroxides.
IDENTIFICATION
First identification: A, B.
Second identification: C, D.
A. Infrared absorption spectrophotometry (2.2.24).
Preparation: Dry the substances before use at 100-105 °C for 3 h.
Comparison: hydrocortisone hydrogen succinate CRS.
B. Thin-layer chromatography (2.2.27).
Solvent mixture methanol R, methylene chloride R (1:9 V/V).
Test solution: Dissolve 10 mg of the substance to be examined in the solvent mixture and dilute to 10 mL with the solvent mixture.
Reference solution (a): Dissolve 20 mg of hydrocortisone hydrogen succinate CRS in the solvent mixture and dilute to 20 mL with the solvent mixture.
Reference solution (b): Dissolve 10 mg of methylprednisolone hydrogen succinate CRS in reference solution (a) and dilute to 10 mL with reference solution (a).
Plate: TLC silica gel F254 plate R.
Mobile phase: anhydrous formic acid R, anhydrous ethanol R, methylene chloride R (0.1:1:15 V/V/V).
Application: 5 μL.
Development: Over a path of 15 cm.
Drying: In air.
Detection A: Examine in ultraviolet light at 254 nm.
Results A: The principal spot in the chromatogram obtained with the test solution is similar in position and size to the principal spot in the chromatogram obtained with reference solution (a).
Detection B Spray with alcoholic solution of sulfuric acid R. Heat at 120 °C for 10 min or until the spots appear. Allow to cool. Examine in daylight and in ultraviolet light at 365 nm.
Results B: The principal spot in the chromatogram obtained with the test solution is similar in position, colour in daylight, fluorescence in ultraviolet light at 365 nm and size to the principal spot in the chromatogram obtained with reference solution (a).
System suitability: Reference solution (b):
— the chromatogram shows 2 spots which may, however, not be completely separated.
C. Thin-layer chromatography (2.2.27).
Test solution (a): Dissolve 25 mg of the substance to be examined in methanol R with gentle heating and dilute to 5 mL with the same solvent (solution A).
Dilute 2 mL of this solution to 10 mL with methylene chloride R. Test solution (b) Transfer 2 mL of solution A to a 15 mL glass tube with a ground-glass stopper or a polytetrafluoroethylene cap. Add 10 mL of a 0.8 g/L solution of sodium hydroxide R in methanol R and immediately pass a stream of nitrogen R briskly through the solution for 5 min. Stopper the tube. Heat in a water-bath at 45 °C, protected from light, for 30 min. Allow to cool.
Reference solution (a): Dissolve 25 mg of hydrocortisone hydrogen succinate CRS in methanol R with gentle heating and dilute to 5 mL with the same solvent (solution B). Dilute 2 mL of this solution to 10 mL with methylene chloride R.
Reference solution (b): Transfer 2 mL of solution B to a 15 mL glass tube with a ground-glass stopper or a polytetrafluoroethylene cap. Add 10 mL of a 0.8 g/L solution of sodium hydroxide R in methanol R and immediately pass a stream of nitrogen R briskly through the solution for 5 min. Stopper the tube.
Heat in a water-bath at 45 °C, protected from light, for 30 min. Allow to cool.
Plate: TLC silica gel F254 plate R.ư
Mobile phase: Add a mixture of 1.2 volumes of water R and 8 volumes of methanol R to a mixture of 15 volumes of ether R and 77 volumes of methylene chloride R.
Application: 5 μL.
Developpement: Over a path of 15 cm.
Drying: In air.
Detection A: Examine in ultraviolet light at 254 nm.
Results A: The principal spot in each of the chromatograms obtained with the test solutions is similar in position and size to the principal spot in the chromatogram obtained with the corresponding reference solution.
Detection B: Spray with alcoholic solution of sulfuric acid R. Heat at 120 °C for 10 min or until the spots appear. Allow to cool. Examine in daylight and in ultraviolet light at 365 nm.
Results B: The principal spot in each of the chromatograms obtained with the test solutions is similar in position, colour in daylight, fluorescence in ultraviolet light at 365 nm and size to the principal spot in the chromatogram obtained with the corresponding reference solution. The principal spot in each of the chromatograms obtained with test solution (b) and reference solution (b) has an RF value distinctly higher than that of the principal spot in each of the chromatograms obtained with test solution (a) and reference solution (a).
D. Add about 2 mg to 2 mL of sulfuric acid R and shake to dissolve. Within 5 min, an intense brownish-red colour develops with a green fluorescence which is particularly intense when viewed in ultraviolet light at 365 nm. Add this solution to 10 mL of water R and mix. The colour fades and a clear solution remains. The fluorescence in ultraviolet light does not disappear.
TESTS
Appearance of solution
The solution is clear (2.2.1).
Dissolve 0.10 g in 5 mL of sodium hydrogen carbonate solution R.
Specific optical rotation (2.2.7)
+ 147 to + 153 (dried substance).
Dissolve 0.250 g in anhydrous ethanol R and dilute to 25.0 mL with the same solvent.
Related substances
Liquid chromatography (2.2.29).
Test solution: Dissolve 25.0 mg of the substance to be examined in a mixture of equal volumes of acetonitrile R and water R and dilute to 10.0 mL with the same mixture of solvents.
Reference solution (a): Dissolve 2 mg of hydrocortisone hydrogen succinate CRS and 2 mg of dexamethasone CRS in 50 mL of acetonitrile R, then dilute to 100.0 mL with water R.
Reference solution (b): Dilute 1.0 mL of the test solution to 100.0 mL with a mixture of equal volumes of acetonitrile R and water R.
Column:
— size: l = 0.25 m, Ø = 4.6 mm;
— stationary phase: octadecylsilyl silica gel for chromatography R (5 μm).
Mobile phase: In a 1000 mL volumetric flask mix 330 mL of acetonitrile R with 600 mL of water R and 1.0 mL of phosphoric acid R, then allow to equilibrate; dilute to 1000 mL with water R and mix again.
Flow rate: 1 mL/min.
Detection: Spectrophotometer at 254 nm.
Equilibration: With the mobile phase for about 30 min.
Injection: 20 μL.
Run time: Twice the retention time of hydrocortisone hydrogen succinate.
Retention time: Dexamethasone = about 12.5 min; hydrocortisone hydrogen succinate = about 15 min.
System suitability: Reference solution (a):
— resolution: minimum 5.0 between the peaks due to dexamethasone and hydrocortisone hydrogen succinate; if necessary, adjust the concentration of acetonitrile in the mobile phase.
Limits:
— impurities A, B: for each impurity, not more than 0.5 times the area of the principal peak in the chromatogram obtained with reference solution (b) (0.5 per cent);
— total: not more than 0.75 times the area of the principal peak in the chromatogram obtained with reference solution (b) (0.75 per cent);
— disregard limit: 0.05 times the area of the principal peak in the chromatogram obtained with reference solution (b) (0.05 per cent).
Loss on drying (2.2.32)
Maximum 4.0 per cent, determined on 1.000 g by drying in an oven at 105 °C.
Sulfated ash (2.4.14)
Maximum 0.1 per cent, determined on 1.0 g.
ASSAY
Dissolve 0.100 g in ethanol (96 per cent) R and dilute to 100.0 mL with the same solvent. Dilute 2.0 mL of this solution to 100.0 mL with ethanol (96 per cent) R. Measure the absorbance (2.2.25) at the absorption maximum at 241.5 nm.
Calculate the content of C25H34O8 taking the specific absorbance to be 353.
STORAGE
In an airtight container, protected from light.
IMPURITIES
Specified impurities A, B.
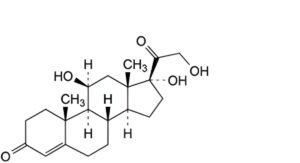
A. 11β,17,21-trihydroxypregn-4-ene-3,20-dione (hydrocortisone),
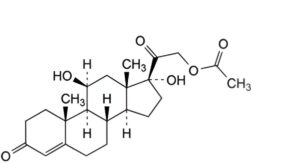
B. 11β,17-dihydroxy-3,20-dioxopregn-4-en-21-yl acetate (hydrocortisone acetate).