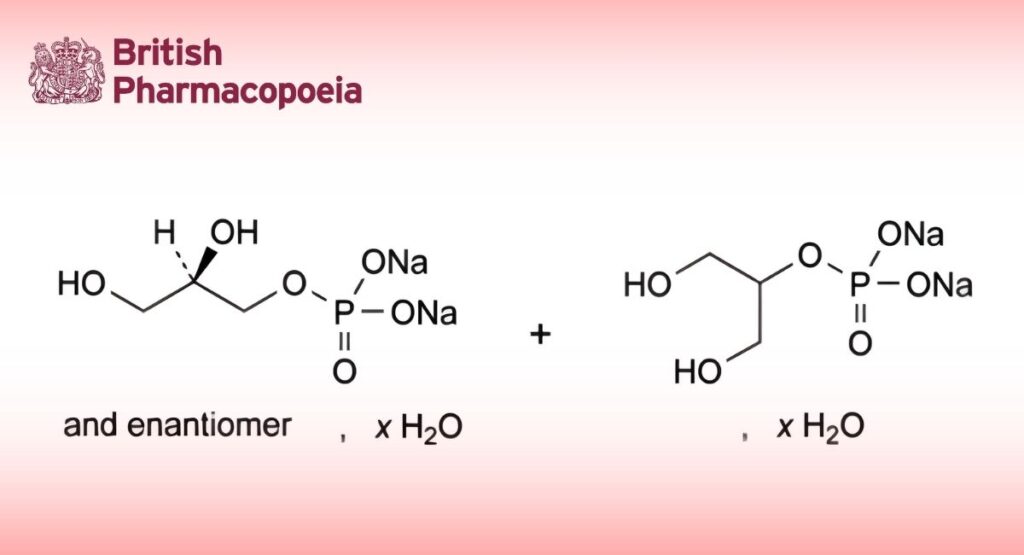
(Ph. Eur. monograph 1995)
C3H7Na2O6P,xH2O 216.0 (anhydrous substance)
DEFINITION
Mixture of variable proportions of hydrated disodium (2RS)-2,3-dihydroxypropyl phosphate and hydrated disodium 2-hydroxy-1-(hydroxymethyl)ethyl phosphate. The mixture may contain various amounts of other glycerophosphate esters.
Content
98.0 per cent to 105.0 per cent (anhydrous substance).
CHARACTERS
Appearance
White or almost white, crystalline powder or crystals.
Solubility
Freely soluble in water, practically insoluble in acetone and in ethanol (96 per cent).
IDENTIFICATION
A. Solution S (see Tests) gives reaction (a) of sodium (2.3.1).
B. To 0.1 g add 5 mL of dilute nitric acid R. Heat to boiling and boil for 1 min. Cool. The solution gives reaction (b) of phosphates (2.3.1).
C. In a test-tube fitted with a glass tube, mix 0.1 g with 5 g of potassium hydrogen sulfate R. Heat strongly and direct the white vapour into 5 mL of decolorised fuchsin solution R. A violet-red colour develops which becomes violet upon heating for 30 min on a water-bath.
TESTS
Solution S
Dissolve 10.0 g in carbon dioxide-free water R prepared from distilled water R and dilute to 100 mL with the same solvent.
Appearance of solution
Solution S is not more opalescent than reference suspension II (2.2.1) and not more intensely coloured than reference solution Y6 (2.2.2, Method II).
Alkalinity
To 10 mL of solution S add 0.2 mL of phenolphthalein solution R. Not more than 1.0 mL of 0.1 M hydrochloric acid is required to change the colour of the indicator (n2).
Glycerol and ethanol (96 per cent)-soluble substances
Maximum 1.0 per cent.
Shake 1.000 g with 25 mL of ethanol (96 per cent) R for 10 min. Filter. Evaporate the filtrate on a water-bath and dry the residue at 70 °C for 1 h. The residue weighs not more than 10 mg.
Chlorides (2.4.4)
Maximum 200 ppm.
Dilute 2.5 mL of solution S to 15 mL with water R.
Phosphates (2.4.11)
Maximum 0.1 per cent.
Dilute 1 mL of solution S to 10 mL with water R. Dilute 1 mL of this solution to 100 mL with water R.
Sulfates (2.4.13)
Maximum 500 ppm.
Dilute 3 mL of solution S to 15 mL with water R.
Calcium
Maximum 100 ppm, if intended for use in the manufacture of parenteral preparations.
Inductively coupled plasma-atomic emission spectrometry (2.2.57).
Test solution Dissolve 0.50 g in a 1 per cent V/V solution of nitric acid R and dilute to 50.0 mL with the same solution.
Reference solutions Prepare the reference solutions using calcium standard solution (100 ppm Ca) R, diluting with a 1 per cent V/V solution of nitric acid R.
Wavelength 396.8 nm.
Iron (2.4.9)
Maximum 20 ppm.
Dilute 5 mL of solution S to 10 mL with water R.
Water (2.5.12)
25.0 per cent to 35.0 per cent, determined on 0.100 g.
ASSAY
Dissolve 0.250 g in 30 mL of water R. Titrate with 0.05 M sulfuric acid, determining the end-point potentiometrically (2.2.20), (n1).
Calculate the percentage content of sodium glycerophosphate (anhydrous substance) using the following expression:
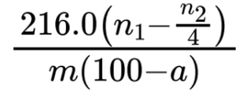
a = percentage content of water;
n1 = volume of 0.05 M sulfuric acid used in the assay, in millilitres;
n2 = volume of 0.1 M hydrochloric acid used in the test for alkalinity, in millilitres;
m = mass of the substance to be examined, in grams.