(Ph. Eur. monograph 2163)
C3H7MnO6P,xH2O 225.0 (anhydrous substance)
DEFINITION
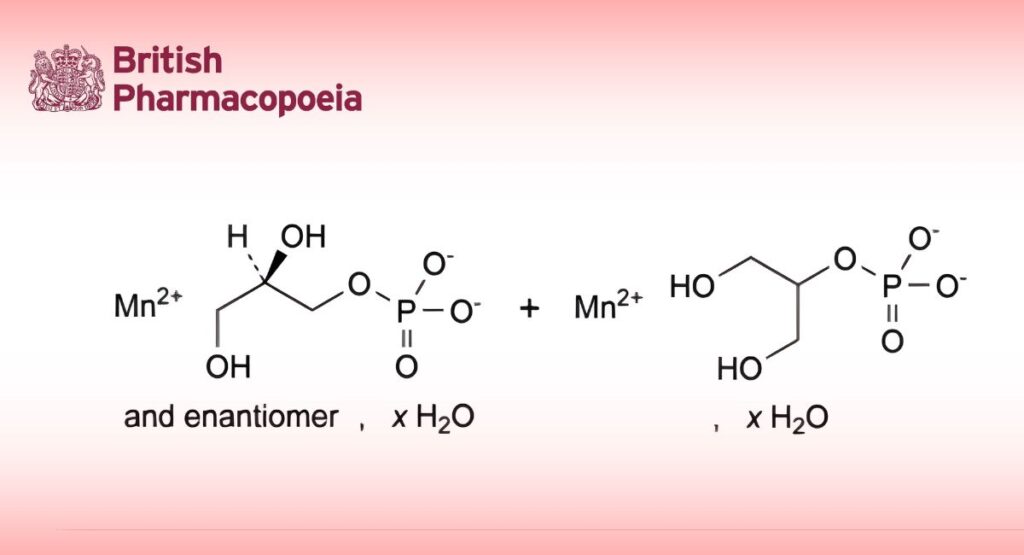
Mixture of variable proportions of hydrated manganese(II) (2RS)-2,3-dihydroxypropyl phosphate and
hydrated manganese(II) 2-hydroxy-1-(hydroxymethyl)ethyl phosphate.
Content
97.0 per cent to 102.0 per cent (dried substance).
CHARACTERS
Appearance
White or pale pink, hygroscopic powder.
Solubility
Practically insoluble in water and in ethanol (96 per cent). It is freely soluble in dilute mineral acids.
IDENTIFICATION
A. Mix 1 g with 1 g of potassium hydrogen sulfate R in a test tube fitted with a delivery tube. Heat strongly
and direct the white vapour towards a piece of filter paper impregnated with a freshly prepared 10 g/L
solution of sodium nitroprusside R. The filter paper develops a blue colour in contact with piperidine R.
B. Disperse 50 mg in 5 mL of water R. Add 0.5 mL of ammonium sulfide solution R. A pale pink precipitate
is formed that dissolves on the addition of 1 mL of acetic acid R.
C. Ignite 0.1 g in a crucible. Take up the residue with 5 mL of nitric acid R and heat on a water-bath for
1 min. Filter. The filtrate gives reaction (b) of phosphates (2.3.1).
TESTS
Solution S
Dissolve 5.0 g in 20 mL of dilute hydrochloric acid R. Filter if necessary. Add dilute ammonia R1 until a
precipitate is formed. Dissolve the precipitate by adding the minimum quantity needed of dilute hydrochloric
acid R and dilute to 100 mL with distilled water R.
Glycerol and ethanol (96 per cent)-soluble substances
Maximum 1.0 per cent.
Shake 1.00 g with 25 mL of ethanol (96 per cent) R for 1 min. Filter. Evaporate the filtrate to dryness on a
water-bath and dry the residue at 70 °C for 1 h. The residue weighs a maximum of 10 mg.
Chlorides (2.4.4)
Maximum 0.15 per cent.
Dissolve 0.22 g in a mixture of 1 mL of nitric acid R and 10 mL of water R and dilute to 100 mL with water R.
Phosphates (2.4.11)
Maximum 0.3 per cent.
Dilute 1.0 mL of solution S to 100.0 mL with water R. To 10 mL of this solution add 140 mL of water R.
Sulfates (2.4.13)
Maximum 0.2 per cent.
Dilute 5 mL of solution S to 50 mL with distilled water R.
Iron (2.4.9)
Maximum 50 ppm.
Dilute 4 mL of solution S to 10 mL with water R.
Loss on drying (2.2.32)
Maximum 12.0 per cent, determined on 1.000 g by drying in an oven at 105 °C for 4 h.
ASSAY
To 0.200 g add 1.5 mL of 1 M hydrochloric acid, 50 mL of water R, 10 mg of ascorbic acid R and 20 mL of
ammonium chloride buffer solution pH 10.0 R. Stir until dissolution. Immediately add 0.3 mL of a 2 g/L
solution of mordant black 11 R in triethanolamine R and titrate with 0.1 M sodium edetate until the colour
changes from violet to pure blue.
1 mL of 0.1 M sodium edetate is equivalent to 22.50 mg of C3H7MnO6P.
STORAGE
In an airtight container.