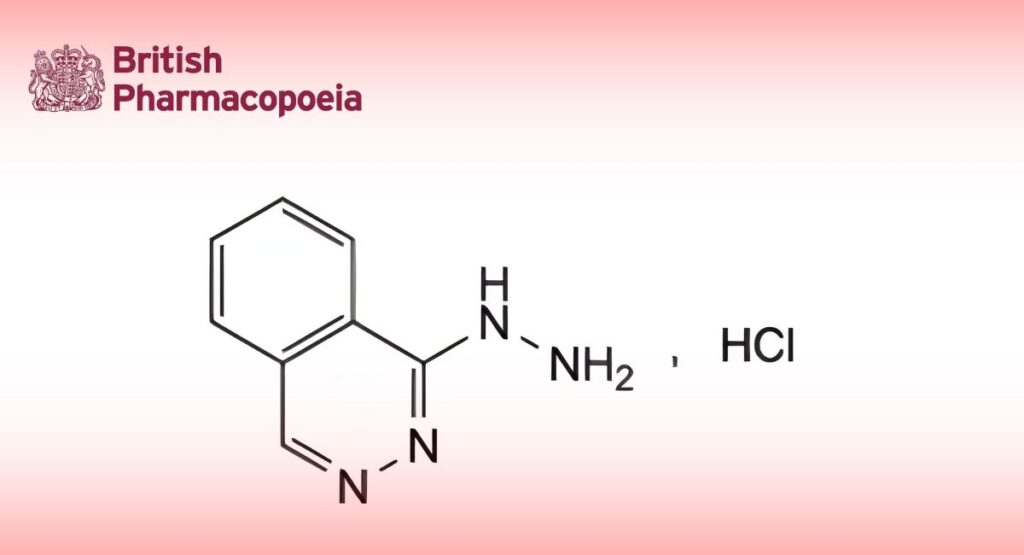
(Ph. Eur. monograph 0829)
C8H9ClN4 196.6 304-20-1
Action and use
Vasodilator; treatment of hypertension.
Preparations
Hydralazine Injection
Hydralazine Tablets
DEFINITION
1-Hydrazinylphthalazine hydrochloride.
Content
98.5 per cent to 101.0 per cent (dried substance).
CHARACTERS
Appearance
White or almost white, crystalline powder.
Solubility
Soluble in water, slightly soluble in ethanol (96 per cent), very slightly soluble in methylene chloride.
mp
About 275 °C, with decomposition.
IDENTIFICATION
First identification: A, D,.
Second identification: B, C, D.
A. Infrared absorption spectrophotometry (2.2.24).
Comparison hydralazine hydrochloride CRS.
B. Dissolve 0.5 g in a mixture of 8 mL of dilute hydrochloric acid R and 100 mL of water R. Add 2 mL of sodium nitrite solution R, allow to stand for 10 min and filter. The precipitate, washed with water R and dried at 100-105 °C, melts (2.2.14) at 209 °C to 212 °C.
C. Dissolve about 10 mg in 2 mL of water R. Add 2 mL of a 20 g/L solution of nitrobenzaldehyde R in ethanol (96 per cent) R. An orange precipitate is formed.
D. Dissolve 11 mg in 2 mL of water R, acidify with 1 drop of dilute sulfuric acid R and add 1 mL of a 6.25 g/L solution of silver sulfate R. Shake and allow to stand. A curdled, white precipitate is formed. Centrifuge and wash the precipitate with 3 quantities, each of 1 mL, of a 0.5 per cent V/V solution of sulfuric acid R. Carry out this operation rapidly in subdued light, disregarding the fact that the supernatant may not become perfectly clear. Suspend the precipitate in 1 mL of water R and add 0.2 mL of ammonia R. Shake vigorously for at least 1 min. The precipitate dissolves easily with the possible exception of a few large particles that dissolve slowly.
TESTS
Solution S
Dissolve 0.5 g in carbon dioxide-free water R and dilute to 25 mL with the same solvent.
Appearance of solution
The solution is clear (2.2.1) and not more intensely coloured than reference solution GY6 (2.2.2, Method II).
Dilute 4 mL of solution S to 20 mL with water R.
pH (2.2.3)
3.5 to 4.2 for solution S.
Impurity B
Liquid chromatography (2.2.29).
Solvent mixture water R, acetonitrile R (30:70 V/V).
Solution A: Dilute 1 mL of benzaldehyde R to 100 mL with a mixture of 10 volumes of water R and 90 volumes of
methanol R.
Test solution: Dissolve 20.0 mg of the substance to be examined in 1 mL of water R and dilute to 5.0 mL with solution A.
Shake for 20 min to allow completion of the derivatisation reaction (hydrazine (impurity B) reacts with benzaldehyde to give benzaldehyde azine). Dilute 2.0 mL of the solution to 5.0 mL with the solvent mixture.
Reference solution: Dissolve 65.0 mg of hydrazine dihydrochloride R (impurity B) in water R and dilute to 100.0 mL with the same solvent. Dilute 1.0 mL of the solution to 100.0 mL with water R. Dilute 5.0 mL of this solution to 100.0 mL with water R. Dilute 1.0 mL of this solution to 5.0 mL with solution A. Shake for 20 min to allow completion of the derivatisation reaction (hydrazine (impurity B) reacts with benzaldehyde to give benzaldehyde azine). Dilute 2.0 mL of this solution to 5.0 mL with the solvent mixture.
Column:
— size: l = 0.25 m, Ø = 4.6 mm;
— stationary phase: octadecylsilyl silica gel for chromatography R (5 μm).
Mobile phase 1.0 g/L solution of sodium edetate R, acetonitrile R (30:70 V/V).
Flow rate 1.0 mL/min.
Detection Spectrophotometer at 310 nm.
Injection 20 μL.
Run time 1.5 times the retention time of benzaldehyde azine.
Retention time Benzaldehyde azine = about 12 min.
System suitability Reference solution:
— signal-to-noise ratio: minimum 20 for the peak due to benzaldehyde azine.
Calculation of content:
— for impurity B, use the concentration of hydrazine dihydrochloride in the reference solution and the peak areas due to benzaldehyde azine.
Limit:
— impurity B: maximum 10 ppm.
Related substances
Liquid chromatography (2.2.29). Prepare the solutions immediately before use.
Solution A 6.0 g/L solution of glacial acetic acid R.
Buffer solution: Dissolve 0.97 g of tetrabutylammonium bromide R and 1.87 g of sodium laurilsulfate R in water for chromatography R and dilute to 1000 mL with the same solvent. Adjust to pH 3.0 with a 9.81 g/L solution of sulfuric acid R.
Test solution: Dissolve 25.0 mg of the substance to be examined in solution A and dilute to 50.0 mL with solution A.
Reference solution (a): Dilute 1.0 mL of the test solution to 100.0 mL with solution A. Dilute 1.0 mL of this solution to 10.0 mL with solution A.
Reference solution (b): Dissolve 7.5 mg of 1-chlorophthalazine R (impurity A) in solution A and dilute to 100 mL with solution A. Dilute 1 mL of the solution to 10 mL with solution A. Dilute 1 mL of this solution to 10 mL with the test solution.
Column:
— size: l = 0.25 m, Ø = 4.6 mm;
— stationary phase: diisopropylcyanosilyl silica gel for chromatography R (5 μm).
Mobile phase acetonitrile for chromatography R, buffer solution (23:77 V/V).
Flow rate 1.0 mL/min.
Detection Spectrophotometer at 230 nm.
Injection 20 μL.
Run time Twice the retention time of hydralazine.
Identification of impurities Use the chromatogram obtained with reference solution (b) to identify the peak due to
impurity A.
Relative retention With reference to hydralazine (retention time = about 13 min): impurity A = about 0.8.
System suitability Reference solution (b):
— resolution: minimum 4.0 between the peaks due to impurity A and hydralazine.
Calculation of percentage contents:
— correction factor: multiply the peak area of impurity A by 0.6;
— for each impurity, use the concentration of hydralazine hydrochloride in reference solution (a).
Limits:
— impurity A: maximum 0.15 per cent;
— unspecified impurities: for each impurity, maximum 0.10 per cent;
— total: maximum 0.3 per cent;
— reporting threshold: 0.05 per cent.
Loss on drying (2.2.32)
Maximum 0.5 per cent, determined on 1.000 g by drying in vacuo.
Sulfated ash (2.4.14)
Maximum 0.1 per cent, determined on 1.0 g.
ASSAY
Dissolve 80.0 mg in 25 mL of water R. Add 35 mL of hydrochloric acid R and titrate with 0.05 M potassium iodate, determining the end-point potentiometrically (2.2.20) using a platinum indicator electrode.
1 mL of 0.05 M potassium iodate is equivalent to 9.832 mg of C8H9ClN4.
STORAGE
Protected from light.
IMPURITIES
Specified impurities A, B.
Other detectable impurities (the following substances would, if present at a sufficient level, be detected by one or other of the tests in the monograph. They are limited by the general acceptance criterion for other/unspecified impurities and/or by the general monograph Substances for pharmaceutical use (2034). It is therefore not necessary to identify these impurities for demonstration of compliance. See also 5.10. Control of impurities in substances for pharmaceutical use) C, D.
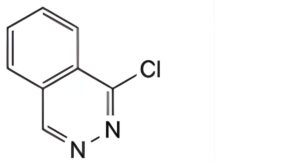
A. 1-chlorophthalazine,
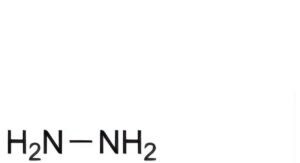
B. hydrazine,
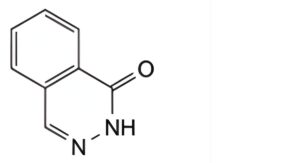
C. phthalazin-1(2H)-one,
D. phthalazine.