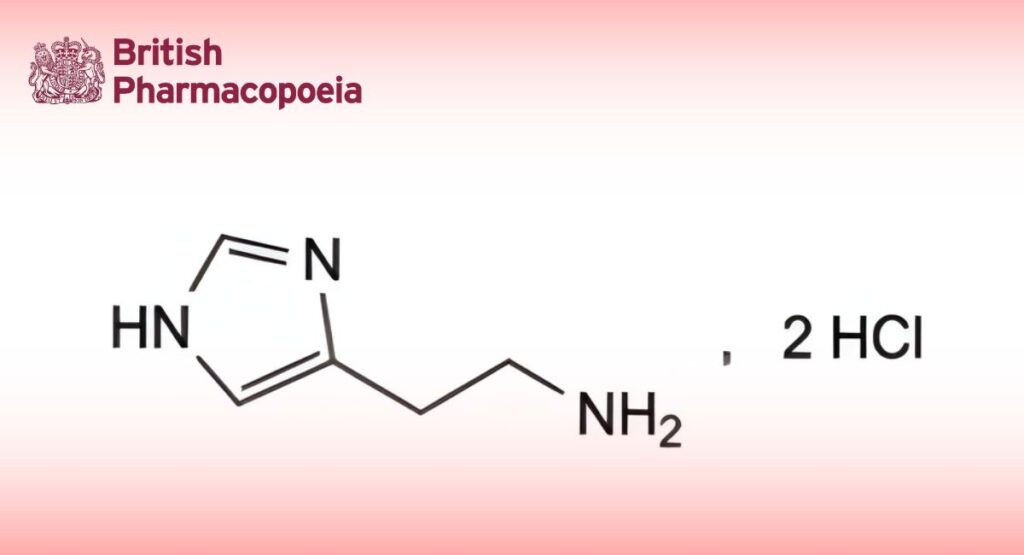
(Ph. Eur. monograph 0143)
C5H11Cl2N3 184.1 56-92-8
DEFINITION
Histamine dihydrochloride contains not less than 98.5 per cent and not more than the equivalent of 101.0 per cent of 2-(1H-imidazol-4-yl)ethan-1-amine dihydrochloride, calculated with reference to the dried substance.
CHARACTERS
A white or almost white, crystalline powder or colourless crystals, hygroscopic, very soluble in water, soluble in ethanol (96 per cent).
IDENTIFICATION
First identification: A, D.
Second identification: B, C, D.
A. Examine by infrared absorption spectrophotometry (2.2.24), comparing with the spectrum obtained with histamine dihydrochloride CRS. Examine as discs prepared using 1 mg of substance.
B. Examine the chromatograms obtained in the test for histidine. The principal spot in the chromatogram obtained with test solution (b) is similar in position, colour and size to the principal spot in the chromatogram obtained with reference solution (a).
C. Dissolve 0.1 g in 7 mL of water R and add 3 mL of a 200 g/L solution of sodium hydroxide R. Dissolve 50 mg of sulfanilic acid R in a mixture of 0.1 mL of hydrochloric acid R and 10 mL of water R and add 0.1 mL of sodium nitrite solution R. Add the second solution to the first and mix. A red colour is produced.
D. It gives reaction (a) of chlorides (2.3.1).
TESTS
Solution S
Dissolve 0.5 g in carbon dioxide-free water R prepared from distilled water R and dilute to 10 mL with the same solvent.
Appearance of solution
Solution S is clear (2.2.1) and not more intensely coloured than reference solution Y7 (2.2.2, Method II).
pH (2.2.3)
The pH of solution S is 2.85 to 3.60.
Histidine
Examine by thin-layer chromatography (2.2.27), using a TLC silica gel G plate R.
Test solution (a): Dissolve 0.5 g of the substance to be examined in water R and dilute to 10 mL with the same solvent.
Test solution (b): Dilute 2 mL of test solution (a) to 10 mL with water R.
Reference solution (a): Dissolve 0.1 g of histamine dihydrochloride CRS in water R and dilute to 10 mL with the same solvent.
Reference solution (b): Dissolve 50 mg of histidine monohydrochloride R in water R and dilute to 100 mL with the same solvent.
Reference solution (c): Mix 1 mL of test solution (a) and 1 mL of reference solution (b).
Apply to the plate 1 μL of test solution (a), 1 μL of test solution (b), 1 μL of reference solution (a), 1 μL of reference solution (b) and 2 μL of reference solution (c). Develop over a path of 15 cm using a mixture of 5 volumes of concentrated ammonia R, 20 volumes of water R and 75 volumes of acetonitrile R. Dry the plate in a current of air. Repeat the development in the same direction, dry the plate in a current of air and spray with ninhydrin solution R1. Heat the plate at 110 °C for 10 min. Any spot corresponding to histidine in the chromatogram obtained with test solution (a) is not more intense than the spot in the chromatogram obtained with reference solution (b) (1 per cent). The test is not valid unless the chromatogram obtained with reference solution (c) shows 2 clearly separated spots.
Sulfates (2.4.13)
3 mL of solution S diluted to 15 mL with distilled water R complies with the limit test for sulfates (0.1 per cent).
Loss on drying (2.2.32)
Not more than 0.5 per cent, determined on 0.20 g by drying in an oven at 105 °C.
Sulfated ash (2.4.14)
Not more than 0.1 per cent, determined on 0.5 g.
ASSAY
Dissolve 0.080 g in a mixture of 5.0 mL of 0.01 M hydrochloric acid and 50 mL of alcohol R. Carry out a potentiometric titration (2.2.20), using 0.1 M sodium hydroxide. Read the volume added between the first and third points of inflexion.
1 mL of 0.1 M sodium hydroxide is equivalent to 9.203 mg of C5H11Cl2N3.
STORAGE
Store in an airtight container, protected from light.