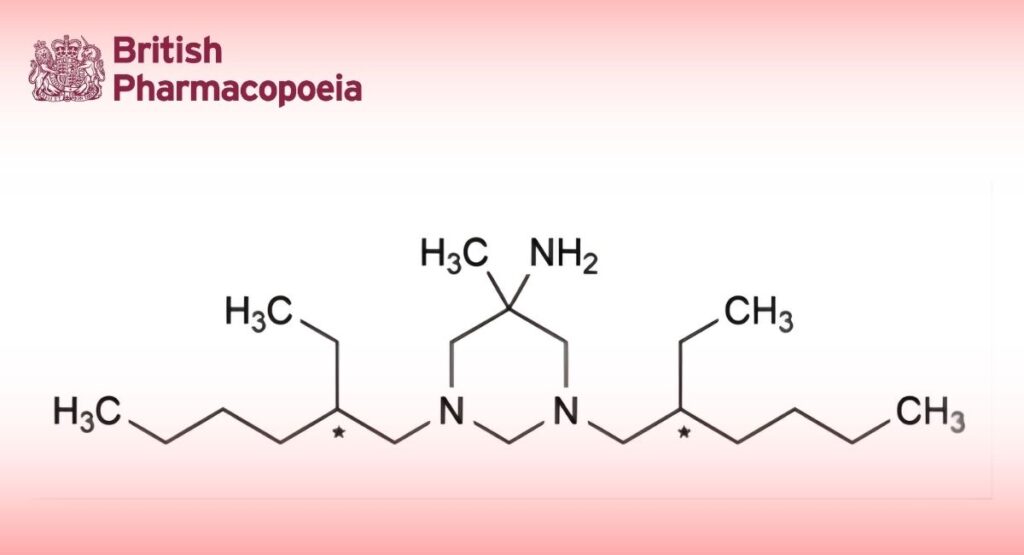
(Ph. Eur. monograph 1221)
C21H45N3 339.6 141-94-6
Action and use
Antiseptic.
DEFINITION
Hexetidine contains not less than 98.0 per cent and not more than the equivalent of 102.0 per cent of 1,3-bis(2-ethylhexyl)]-5-methylhexahydropyrimidin-5-amine.
CHARACTERS
An oily liquid, colourless or slightly yellow, very slightly soluble in water, very soluble in acetone, in ethanol (96 per cent) and in methylene chloride. It dissolves in dilute mineral acids.
IDENTIFICATION
First identification: A.
Second identification: B, C, D.
A. Examine by infrared absorption spectrophotometry (2.2.24), comparing with the spectrum obtained with hexetidine CRS.
B. Examine the chromatograms obtained in the test for related substances. The principal spot in the chromatogram obtained with test solution (b) is similar in position, colour and size to the principal spot in the chromatogram obtained with reference solution (a).
C. To 0.2 mL add 2 mL of sulfuric acid R and 2 mg of chromotropic acid, sodium salt R. Heat in a water-bath at 60 °C. A violet colour develops.
D. Dissolve 0.2 mL in 1 mL of methylene chloride R. Add 0.5 mL of copper sulfate solution R, 0.05 mL of 0.25 M alcoholic sulfuric acid R and 5 mL of water
R. Shake, then allow to stand. The lower layer becomes deep blue.
TESTS
Appearance
The substance to be examined is clear (2.2.1)and not more intensely coloured than reference solution Y5 or reference solution GY5 (2.2.2, Method II).
Relative density (2.2.5)
0.864 to 0.870.
Refractive index (2.2.6)
1.461 to 1.467.
Optical rotation (2.2.7)
Dissolve 1.0 g in ethanol R and dilute to 10.0 mL with the same solvent. The angle of optical rotation is -0.10° to + 0.10°.
Absorbance (2.2.25)
Dissolve 0.50 g in heptane R and dilute to 50.0 mL with the same solvent. At wavelengths from 270 nm to 350 nm, the absorbance of the solution is not greater than 0.1.
Related substances
Examine by thin-layer chromatography (2.2.27), using silica gel H R as the coating substance. Prepare the solutions immediately before use.
Test solution (a): Dissolve 2.0 g of the substance to be examined in heptane R and dilute to 20 mL with the same solvent.
Test solution (b): Dilute 1 mL of test solution (a) to 10 mL with heptane R.
Reference solution (a): Dissolve 20 mg of hexetidine CRS in heptane R and dilute to 2 mL with the same solvent.
Reference solution (b): Dilute 1 mL of test solution (a) to 100 mL with heptane R.
Reference solution (c): Dilute 5 mL of reference solution (b) to 10 mL with heptane R.
Reference solution (d): Dissolve 10 mg of dehydrohexetidine CRS in test solution (a) and dilute to 10 mL with the same solution.
Apply separately to the plate 1 μL of each solution. At the bottom of a chromatographic tank, place an evaporating dish containing concentrated ammonia R1. Place the dried plate in the tank and close the tank. Leave the plate in contact with the ammonia vapour for 15 min. Withdraw the plate and place it in a current of air to remove the ammonia vapour. Develop over a path of 15 cm using a mixture of 20 volumes of methanol R and 80 volumes of toluene R. Allow the plate to dry in air. Expose the plate to iodine vapour for 30 min. Any spot in the chromatogram obtained with test solution (a), apart from the principal spot, is not more intense than the spot in the chromatogram obtained with reference solution (b) (1 per cent) and at most two such spots are more intense than the spot in the chromatogram obtained with reference solution (c) (0.5 per cent). The test is not valid unless the chromatogram obtained with reference solution (d) shows two clearly separated spots.
Sulfated ash (2.4.14)
Not more than 0.1 per cent, determined on 1.0 g.
ASSAY
Dissolve 0.150 g in 80 mL of anhydrous acetic acid R. Titrate with 0.1 M perchloric acid, determining the end-point potentiometrically (2.2.20).
1 mL of 0.1 M perchloric acid is equivalent to 16.98 mg of C21H45N3.
STORAGE
Store protected from light.
IMPURITIES
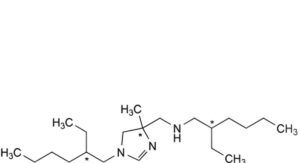
A. 2-ethyl-N-[[1-(2-ethylhexyl)-4-methyl-4,5-dihydro-1H-imidazol-4-yl]methyl]hexan-1-amine (dehydrohexetidine),
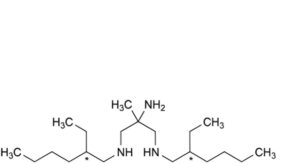
B. N ,N -bis(2-ethylhexyl)-2-methylpropane-1,2,3-triamine (triamine),
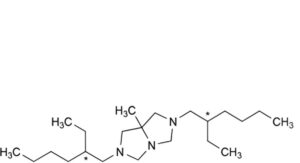
C. 2,6-bis(2-ethylhexyl)-7a-methylhexahydro-1H-imidazo[1,5-c]imidazole (hexedine),
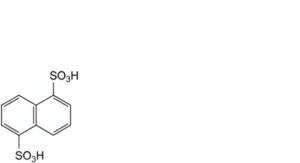
D. naphthalene-1,5-disulfonic acid.