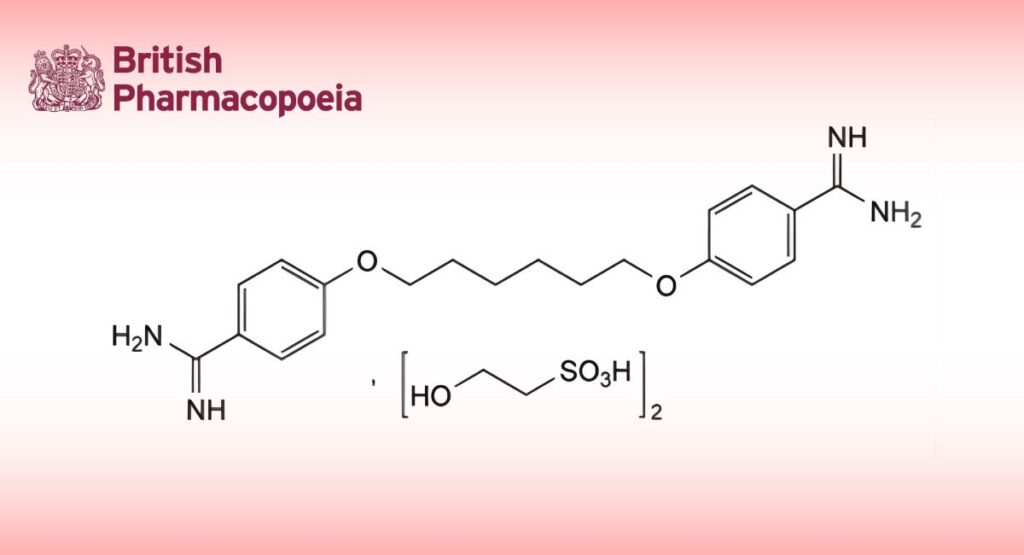
(Hexamidine Diisetionate, Ph. Eur. monograph 1436)
C24H38N4O10S2 607 659-40-5
Action and use
Antiprotozoal.
DEFINITION
4,4′-[Hexane-1,6-diylbis(oxy)]dibenzimidamide bis(2-hydroxyethanesulfonate).
Content
98.5 per cent to 101.5 per cent (dried substance).
PRODUCTION
It is considered that alkyl 2-hydroxyethanesulfonate esters are potential impurities in hexamidine diisetionate. The manufacturing process should be developed taking into consideration the principles of quality risk management, together with considerations of the quality of starting materials, process capability and validation including, where necessary, demonstration that alkyl 2-hydroxyethanesulfonate esters are not detectable in the final product.
CHARACTERS
Appearance
White or slightly yellow powder, hygroscopic.
Solubility
Sparingly soluble in water, slightly soluble in ethanol (96 per cent), practically insoluble in methylene chloride.
IDENTIFICATION
A. Infrared absorption spectrophotometry (2.2.24).
Comparison hexamidine diisetionate CRS.
B. Dissolve about 40 mg in 5 mL of water R and add dropwise with shaking 1 mL of a 100 g/L solution of sodium chloride R. Allow to stand for 5 min. An abundant, shimmering white precipitate is slowly formed.
TESTS
Appearance of solution
Dissolve 0.50 g in carbon dioxide-free water R, heating at about 70 °C and dilute to 10 mL with the same solvent. Allow to cool to room temperature for 10-15 min. The solution is not more opalescent than reference suspension II (2.2.1) and not more intensely coloured than intensity 6 of the range of reference solutions of the most appropriate colour (2.2.2, Method II).
Acidity or alkalinity
Dissolve 2.0 g in water R heating at about 50 °C and dilute to 20 mL with water R heating at about 50 °C. Allow to cool to about 35 °C, add 0.1 mL of methyl red solution R. Not more than 0.25 mL of 0.05 M hydrochloric acid or 0.05 M sodium hydroxide is required to change the colour of the indicator.
Related substances
Liquid chromatography (2.2.29).
Test solution Dissolve 20.0 mg of the substance to be examined in mobile phase A and dilute to 100.0 mL with mobile phase A.
Reference solution (a) Dilute 1.0 mL of the test solution to 100.0 mL with mobile phase A.
Reference solution (b) Dilute 1.0 mL of reference solution (a) to 10.0 mL with mobile phase A.
Reference solution (c) Dissolve 5 mg of the substance to be examined and 5 mg of pentamidine diisetionate CRS in mobile phase A and dilute to 100 mL with mobile phase A. Dilute 2 mL of the solution to 5 mL with mobile phase A.
Column:
— size: l = 0.25 m, Ø = 4.6 mm;
— stationary phase: styrene-divinylbenzene copolymer R (8 μm).
Mobile phase:
— mobile phase A: mix 20 volumes of acetonitrile R and 80 volumes of a 6.8 g/L solution of potassium dihydrogen phosphate R previously adjusted to pH 3.0 with phosphoric acid R,
— mobile phase B: mix equal volumes of acetonitrile R and of a 6.8 g/L solution of potassium dihydrogen phosphate R previously adjusted to pH 3.0 with phosphoric acid R,
| Time (min) |
Mobile phase A (per cent V/V) |
Mobile phase B (per cent V/V) |
| 0 – 30 | 100 → 0 | 0 → 100 |
| 30 – 35 | 0 | 100 |
| 35 – 40 | 0 → 100 | 100 → 0 |
Flow rate 1 mL/min.
Detection: Spectrophotometer at 263 nm.
Injection 20 μL.
Relative retention: With reference to hexamidine (retention time = about 6 min): impurity B = about 1.7;
impurity A = about 2.0; impurity C = about 3.7; impurity D = about 4.7.
System suitability Reference solution (c):
— resolution: minimum 5.0 between the peaks due to hexamidine and pentamidine.
Limits:
— impurity A: not more than the area of the principal peak in the chromatogram obtained with reference solution (a) (1.0 per cent);
— impurity B: not more than 3 times the area of the principal peak in the chromatogram obtained with reference solution (b) (0.3 per cent);
— impurities C, D: for each impurity, not more than the area of the principal peak in the chromatogram obtained with reference solution (b) (0.1 per cent);
— any other impurities: for each impurity, not more than the area of the principal peak in the chromatogram obtained with reference solution (b) (0.1 per cent);
— total: not more than 1.5 times the area of the principal peak in the chromatogram obtained with reference solution (a) (1.5 per cent);
— disregard limit: 0.5 times the area of the principal peak in the chromatogram obtained with reference solution (b) (0.05 per cent).
Loss on drying (2.2.32)
Maximum 0.5 per cent, determined on 1.000 g by drying in an oven at 105 °C.
Sulfated ash (2.4.14)
Maximum 0.1 per cent, determined on 1.0 g.
ASSAY
Dissolve 0.250 g in 50 mL of dimethylformamide R. Titrate with 0.1 M tetrabutylammonium hydroxide under a current of nitrogen R, determining the end-point potentiometrically (2.2.20).
1 mL of 0.1 M tetrabutylammonium hydroxide is equivalent to 30.35 mg of C24H38N4O10S2.
STORAGE
In an airtight container.
IMPURITIES
Specified impurities A, B, C, D.
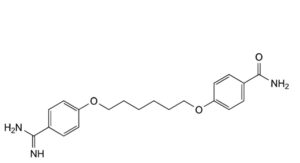
A. 4-[[6-(4-carbamimidoylphenoxy)hexyl]oxy]benzamide,
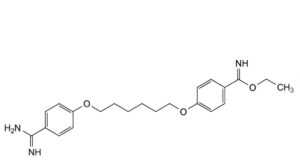
B. ethyl 4-[[6-(4-carbamimidoylphenoxy)hexyl]oxy]benzimidoate,
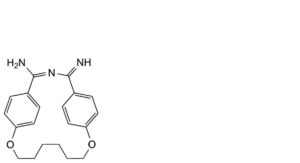
C. 4-imino-9,16-dioxa-3-azatricyclo[15.2.2.2 ]tricosa-1(19),2,5,7,17,20,22-heptaen-2-amine,
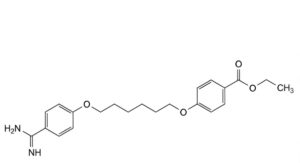
D. ethyl 4-[[6-(4-carbamimidoylphenoxy)hexyl]oxy]benzoate.