BP 2025 (Ph. Eur. 11.6 update)
Action and use
Gonadotrophin-releasing hormone, gonadorelin analogue; treatment of prostate cancer.
DEFINITION
Goserelin Implants are biodegradable and biocompatible cylinders for subcutaneous injection containing Goserelin, as acetate, dispersed in a polymeric matrix. They are formulated so that the medicament is released over a period of weeks.
The implants comply with the requirements stated under Parenteral Preparations and with the following requirements.
Content of goserelin, C59H84N18O14
90.0 to 110.0% of the stated amount of the peptide.
IDENTIFICATION
A. In the test for Uniformity of content, the retention time of the principal peak in the chromatogram obtained with solution (1) is similar to that of the principal peak in the chromatogram obtained with solution (2).
B. In the Assay, the retention time of the principal peak in the chromatogram obtained with solution (1) is similar to that of the principal peak in the chromatogram obtained with solution (2).
TESTS
Drug release
NOTE: Suitable procedures should be employed to minimise the presence of bacteria on glassware immediately prior to the dissolution tests.
For 3.6 mg implants
PROCEDURE
Perform the test in a 120-mL flat-bottomed glass jar with a cap, incubated at 39°± 0.5°. Use as the medium 50 mL of a pH 7.4 buffer solution prepared by dissolving 25.8 g of anhydrous disodium hydrogen orthophosphate, 1.92 g of citric acid and 0.2 g of sodium azide in sufficient distilled water to produce 1000 mL. Adjust the pH of the resulting solution to 7.4 with anhydrous disodium hydrogen orthophosphate or citric acid, if necessary, and filter through a sterile filter with a nominal pore size not greater than 0.2 μm into a sterile container. Place 5 implants into the glass vessel, add 50 mL of the medium and place in the incubator. At the times specified in Table I remove the vessel from the incubator and allow to cool to room temperature. Swirl gently to ensure a uniform solution and withdraw a 5-mL sample. Dilute the sample with 5 mL of the medium, filter if necessary and measure the average of the individual absorbance values obtained at 1 nm intervals between 275 nm and 285 nm, Appendix II B, using the dissolution medium in the reference cell. Add 5 mL of the medium
Goserelin Implants to the dissolution vessel to replace the volume removed and return the vessel to the incubator. At least three replicate
analyses should be performed.
Table 1
| Test Duration Hours (days) | Amount dissolved (each replicate) |
| 168(7) | 2 to 20% |
| 336(14) | 25 to 55% |
| 408(17) | 35 to 75% |
| 504(21) | 65 to 90% |
| 672(28) | 85 to 105% |
DETERMINATION OF CONTENT
Calculate the content of C59H84N18O14 dissolved in the portions of the medium from the absorbance obtained from a solution prepared by diluting a suitable amount of goserelin EPCRS in the dissolution medium and using the declared content of C59H84N18O14 in goserelin EPCRS; at the times specified in Table I the cumulative percentages comply with the criteria given.
For 10.8 mg implants
PROCEDURE
Perform the test using the same apparatus as described for the 3.6 mg strength implant. Use as the medium 50 mL of a phosphate buffered saline pH 7.4 (PBS) solution prepared by mixing 8 g of sodium chloride, 0.19 g of potassium dihydrogen orthophosphate, 1.38 g of anhydrous disodium hydrogen orthophosphate and 0.2 g of sodium azide in 1000 mL of distilled water. Adjust the pH of the solution, if necessary, to 7.4 with 2M hydrochloric acid. Filter the solution through a sterile filter with a nominal pore size not greater than 0.2 μm into a sterile container. Transfer 50 mL of medium to a vessel and heat at 39°± 0.5°. Place a single implant into the vessel and withdraw 5-mL aliquots at 24, 72, 168 and 264 hours (1, 3, 7 and 11 days) and 20-mL aliquots at 336, 504, 672, 840, 1008, 1176, 1344, 1512, 1680, 1848 and 2016 hours (14, 21, 28, 35, 42, 49, 56, 63, 70, 77 and 84 days) following gentle swirling to ensure a uniform solution. Replace the volume removed with the equivalent amount of warmed medium. Filter the aliquot, if necessary, and measure the average of the individual absorbance values obtained at 1 nm intervals between 275 nm and 285 nm, Appendix II B, using the dissolution medium in the reference cell. At least six replicate analyses should be performed.
| Test Duration Hours (days) | Amount dissolved (each replicate) |
| 72(3) | 5 to 30% |
| 336(14) | 10 to 45% |
| 840(35) | 15 to 60% |
| 1344(56) | 55 to 90% |
| 2016(84) | Not less than 70% |
DETERMINATION OF CONTENT
Calculate the content of C59H84N18O14 dissolved in the portions of the medium from the absorbance obtained from a solution prepared by diluting a suitable amount of goserelin EPCRS in the dissolution medium and using the declared content of C59H84N18O14 in goserelin EPCRS. At all time points calculate the cumulative percentage of the labelled amount of C59H84N18O14 dissolved; at the times specified in Table II the cumulative percentages comply with the criteria given.
Related substances
A. Carry out the method for liquid chromatography, Appendix III D, using the following solutions.
(1) Weigh and dissolve 10 implants, with the aid of ultrasound, in a mixture of 15 volumes of water and 85 volumes of acetonitrile to obtain a solution containing 0.2% w/v of Goserelin.
(2) Dilute 1 volume of solution (1) to 100 volumes using a mixture of 15 volumes of water and 85 volumes of acetonitrile.
(3) Dilute 1 volume of solution (2) to 10 volumes using a mixture of 15 volumes of water and 85 volumes of acetonitrile.
(4) 0.002% w/v each of 4-D-Ser-goserelin EPCRS and goserelin EPCRS in water.
CHROMATOGRAPHIC CONDITIONS
(a) Use a stainless steel column (25 cm × 4.6 mm) packed with octadecylsilyl silica gel for chromatography (5 μm) (Spherisorb ODS is suitable).
(b) Use isocratic elution and the mobile phase described below.
(c) Use a flow rate of 1.8 mL per minute.
(d) Use an ambient column temperature.
(e) Use a detection wavelength of 220 nm.
(f) Inject 5 μL of each solution.
MOBILE PHASE
0.272% w/v of potassium dihydrogen orthophosphate in a mixture of 27 volumes of acetonitrile and 73 volumes of water.
Adjust the pH to 3.0 with orthophosphoric acid.
When the chromatograms are recorded under the prescribed conditions, the relative retention with reference to goserelin (retention time about 30 minutes) of 4-D-Ser-goserelin is about 0.84.
SYSTEM SUITABILITY
The test is not valid unless, in the chromatogram obtained with solution (4):
the resolution between the peaks due to 4-D-Ser-goserelin and goserelin is at least 4.5;
the symmetry factor of the peak due to goserelin is less than 2.0.
LIMITS
In the chromatogram obtained with solution (1):
the area of any secondary peak is not greater than the area of the principal peak in the chromatogram obtained with solution (2) (1%);
the sum of the areas of any secondary peaks is not greater than four times the area of the principal peak in the chromatogram obtained with solution (2) (4%).
Disregard any peak with an area less than that of the principal peak in the chromatogram obtained with solution (3) (0.1%).
B. Carry out the method for size-exclusion chromatography, Appendix III C, using the following solutions.
(1) Weigh and dissolve 10 implants, with the aid of ultrasound, in a mixture of 15 volumes of water and 85 volumes of acetonitrile to obtain a solution containing 0.2% w/v of Goserelin.
(2) Dilute 1 volume of solution (1) to 100 volumes using a mixture of 15 volumes of water and 85 volumes of acetonitrile.
(3) Dilute 1 volume of solution (2) to 20 volumes using a mixture of 15 volumes of water and 85 volumes of acetonitrile.
(4) 0.002% w/v each of 4-D-Ser-goserelin EPCRS and goserelin EPCRS in a mixture of 20 volumes of water and 80 volumes of acetonitrile.
CHROMATOGRAPHIC CONDITIONS
(a) Use a stainless steel column (30 cm × 7.8 mm) packed with hydrophilic silica gel for chromatography (5 μm) with a fractionation range for proteins with a relative molecular mass of approximately 4000 to 500 000 (TSK-GEL-G2000 SWXL is suitable).
(b) Use isocratic elution and the mobile phase described below.
(c) Use a flow rate of 1 mL per minute.
(d) Use an ambient column temperature.
(e) Use a detection wavelength of 280 nm.
(f) Inject 5 μL of each solution.
MOBILE PHASE
8 volumes of a solution prepared as described below and 92 volumes of acetonitrile.
To prepare the solution add 167.5 g of a 60% w/v solution of perchloric acid to a 1000-mL volumetric flask, cool in ice and dilute to volume with 1M sodium hydroxide. To 100 mL of this solution add sufficient water to produce 1000 mL and adjust the pH of the solution to 2.1 with 5M sodium hydroxide.
When the chromatograms are recorded using the prescribed conditions the retention times relative to goserelin (retention time about 40 minutes) are: impurity 11, about 0.93; impurity 7 (polymer envelope), about 0.1 to 0.6.
SYSTEM SUITABILITY
The test is not valid unless, in the chromatogram obtained with solution (4), the resolution between the peaks due to 4-D-Ser-goserelin and goserelin is at least 4.0.
LIMITS
In the chromatogram obtained with solution (1):
the area of any peak corresponding to impurity 11 is not greater than twice the area of the principal peak in the chromatogram obtained with solution (2) (2%);
the sum of the areas of any peaks corresponding to the polymer envelope is not greater than 5.5 times the area of the principal peak in the chromatogram obtained with solution (2) (5.5%).
Disregard any peak with an area less than that of the principal peak in the chromatogram obtained with solution (3) (0.05%).
C. Carry out the method for size-exclusion chromatography, Appendix III C, using the following solutions.
(1) Weigh and dissolve 10 implants, with the aid of ultrasound, in a mixture of 15 volumes of water and 85 volumes of acetonitrile to obtain a solution containing 0.2% w/v of Goserelin.
(2) Dilute 1 volume of solution (1) to 100 volumes using a mixture of 15 volumes of water and 85 volumes of acetonitrile.
(3) To 4 mg of goserelin EPCRS add 250 μL of trifluoroacetic acid and leave to stand for 24 hours. Dissolve the product in 20 mL of a mixture of 15 volumes of water and 85 volumes of acetonitrile to obtain a solution containing des-t-butyl-
goserelin.
CHROMATOGRAPHIC CONDITIONS
(a) Use a stainless steel column (30 cm × 7.8 mm) packed with hydrophilic silica gel for chromatography (5 μm) with a fractionation range for proteins with a relative molecular mass of approximately 4000 to 500 000 (TSK-GEL-G2000 SWXL is suitable).
(b) Use isocratic elution and the mobile phase described below.
(c) Use a flow rate of 2 mL per minute.
(d) Use an ambient column temperature.
(e) Use a detection wavelength of 280 nm.
(f) Inject 50 μL of each solution.
MOBILE PHASE
12.5 volumes of a solution prepared as described below and 87.5 volumes of acetonitrile.
Add 167.5 g of a 60% w/v solution of perchloric acid to a 1000-mL volumetric flask, cool in ice and dilute to volume with 1M sodium hydroxide. To 100 mL of this solution add sufficient water to produce 1000 mL and adjust the pH of the solution to 2.1 with 5M sodium hydroxide.
When the chromatograms are recorded under the prescribed conditions the retention time relative to des-t-butyl-goserelin (retention time about 20 minutes) of impurity 13 is about 1.1.
SYSTEM SUITABILITY
The test is not valid unless, in the chromatogram obtained with solution (3), the retention time of des-t-butyl-goserelin is between 19 and 22 minutes.
LIMITS
In the chromatogram obtained with solution (1) the area of the peak corresponding to impurity 13 is not greater than the area of the principal peak in the chromatogram obtained with solution (2) (1%).
The sum of impurities obtained in test B and impurity 13 in test C is not more than 10%.
Acetic acid
Carry out the method for gas chromatography, Appendix III B, using a 2% v/v solution of n-hexadecane (internal standard) in dimethylformamide (solution B) and the following solutions.
(1) Dissolve a quantity of the implant and a quantity of solution B and dilute with sufficient dimethylformamide to obtain a solution containing about 2.8% w/v of Goserelin Implant and 0.1% v/v of n-hexadecane.
(2) Prepare a solution containing 0.625% w/v of glacial acetic acid in dimethylformamide. To 10 mL of this solution add 5 mL of solution B and dilute to 100 mL with dimethylformamide.
CHROMATOGRAPHIC CONDITIONS
(a) Use a free fatty acid phase capillary column (10 m × 0.32 mm) with a nominal film thickness of 0.2 to 0.3 μm (FFAP CB is suitable).
(b) Use helium as the carrier gas at a flow rate of 1.3 mL per minute with a flow rate of the make up gas of 30 mL per minute.
(c) Use the gradient conditions described below.
(d) Use an injection temperature of 200°
(d) Use a flame ionisation detector at a temperature of 250°.
(e) Inject 1 μL of each solution.
(f) Use a split ratio of 30:100.
| Time (Minutes) | Temperature | comment |
| 0→1 | 50o | isothermal |
| 1→6 | 50o→200o | linear gradient |
| 6→9 | 200o | isothermal |
| 9→10 | 200o→50o | linear gradient |
| 10-15 | 50o | re-equilibration |
SYSTEM SUITABILITY
The test is not valid unless, in the chromatogram obtained with solution (2): the resolution between the peaks due to acetic acid and n-hexadecane is at least 15; the symmetry factor of the peak due to acetic acid is less than 2.0.
LIMITS
In the chromatogram obtained with solution (1) the ratio of the area of any peak corresponding to acetic acid to the area of the peak due to the internal standard is not greater than the corresponding ratio in the chromatogram obtained with solution (2) (2.5%).
Water
Not more than 2.5% w/w, Appendix IX C. Use Method III. Prepare the sample by dissolving approximately 110 mg of the implant in 4 mL of dry dimethylformamide.
Bacterial endotoxins
Carry out the test for bacterial endotoxins, Appendix XIV C. Dissolve the copolymer using 2 mL of acetonitrile per implant and dilute to a suitable concentration with water BET (solution A). The endotoxin limit concentration of solution A is 350 IU of endotoxin per implant.
Uniformity of content
The implants comply with the limits described in test A for uniformity of content, Appendix XII C, using the following method of analysis.
Carry out the method for liquid chromatography, Appendix III D, using the following solutions.
(1) Dissolve with the aid of ultrasound, 1 implant in a mixture of 15 volumes of water and 85 volumes of acetonitrile to obtain a solution containing 0.02% w/v of Goserelin.
(2) 0.02% w/v of goserelin EPCRS in a mixture of 15 volumes of water and 85 volumes of acetonitrile.
(3) 0.02% w/v each of goserelin hexapeptide BPCRS and goserelin EPCRS in a mixture of 4 volumes of water and 1 volume of acetonitrile.
CHROMATOGRAPHIC CONDITIONS
(a) Use a stainless steel column (25 cm × 4.6 mm) packed with octadecylsilyl silica gel for chromatography (5 μm) (Spherisorb ODS is suitable).
(b) Use isocratic elution and the mobile phase described below.
(c) Use a flow rate of 1.8 mL per minute.
(d) Use a column temperature of 35°.
(e) Use a detection wavelength of 220 nm.
(f) Inject 10 μL of each solution.
MOBILE PHASE
0.272% w/v of potassium dihydrogen orthophosphate in a mixture of 45 volumes of water and 55 volumes of methanol.
Adjust the pH to 3.0 with orthophosphoric acid.
SYSTEM SUITABILITY
The test is not valid unless, in the chromatogram obtained with solution (3), the resolution between the peaks due to goserelin hexapeptide and goserelin is not less than 4.0.
DETERMINATION OF CONTENT
Calculate the total content of goserelin, C59H84N18O14, from the chromatograms obtained and using the declared content of C59H84N18O14 in goserelin EPCRS.
ASSAY
Carry out the method for size-exclusion chromatography, Appendix III C, using the following solutions.
(1) Weigh and dissolve 10 implants, with the aid of ultrasound, in a mixture of 15 volumes of water and 85 volumes of acetonitrile to obtain a solution containing 0.2% w/v of Goserelin.
(2) 0.2% w/v of goserelin EPCRS in a mixture of 15 volumes of water and 85 volumes of acetonitrile.
(3) 0.002% w/v each of 4-D-Ser-goserelin EPCRS and goserelin EPCRS in a mixture of 20 volumes of water and 80 volumes of acetonitrile.
CHROMATOGRAPHIC CONDITIONS
The chromatographic conditions described under Related substances test B, may be used.
SYSTEM SUITABILITY
The test is not valid unless, in the chromatogram obtained with solution (3), the resolution between the peaks due to 4-D-Ser-goserelin and goserelin is at least 4.0.
DETERMINATION OF CONTENT
Calculate the content of goserelin, C59H84N18O14, from the chromatograms obtained and using the declared content of C59H84N18O14 in goserelin EPCRS.
STORAGE
Goserelin Implants should be stored in a sealed container, protected from light and moisture, at a temperature not exceeding 25°.
LABELLING
The label states the equivalent amount of the peptide in mg in each implant.
IMPURITIES
The impurities controlled by this monograph include:
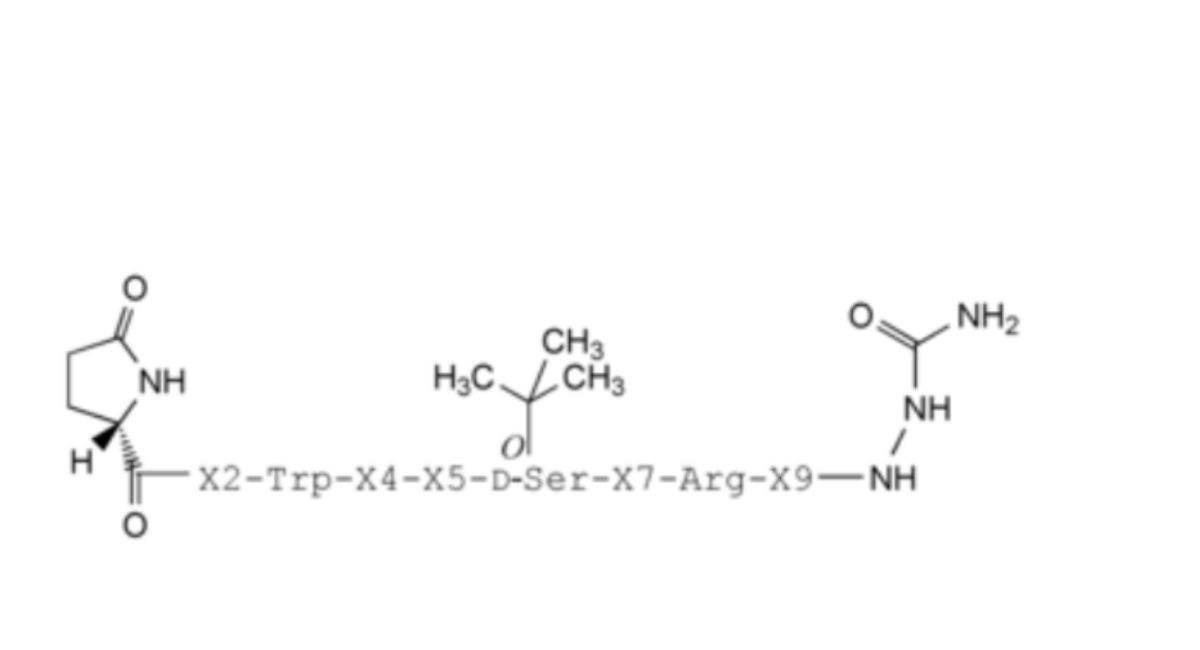
1. X2 = L-His, X4 = D-Ser, X5 = L-Tyr, X7 = L-Leu, X9 = L-Pro: 4(D-serine)-goserelin (equivalent to Ph. Eur. impurity A),
6. X2 = L-His, X4 = L-Ser, X5 = D-Tyr, X7 = L-Leu, X9 = L-Pro: 5(D-tyrosine)-goserelin (equivalent to Ph. Eur. impurity F),
12. X2 = L-His, X4 = L-Ser, X5 = L-Tyr, X7 = D-Leu, X9 = L-Pro: 7(D-leucine)-goserelin (equivalent to Ph. Eur. impurity L),
![6-[O-(1,1-dimethylethyl)-L-serine]-goserelin (equivalent to Ph. Eur. impurity B)](https://nhathuocngocanh.com/bp/wp-content/uploads/2025/10/Thiet-ke-chua-co-ten-2.jpg)
2. 6-[O-(1,1-dimethylethyl)-L-serine]-goserelin (equivalent to Ph. Eur. impurity B),
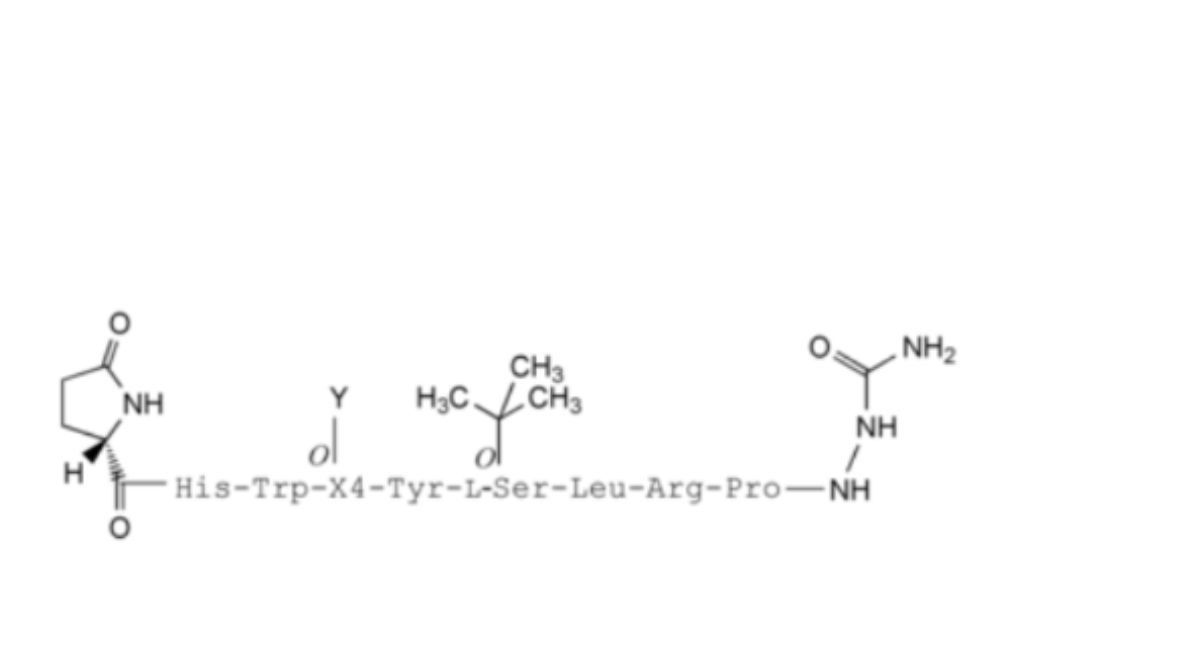
3. X4 = L-Ser, Y = Acetyl: 4-(acetyl-L-serine)-goserelin,
4. X4 = D-Ser, Y = Acetyl: 4-(acetyl-D-serine)-goserelin,
5. X4 = L-Ser, Y = Lactyl: 4-(lactyl-L-serine)-goserelin,
11. X4 = L-Ser, Y = Glycolyl: 4-(glycolyl-L-serine)-goserelin,
7. X4 = L-Ser, Y = Lactide/Glycolide copolymer chain: Polymer envelope,
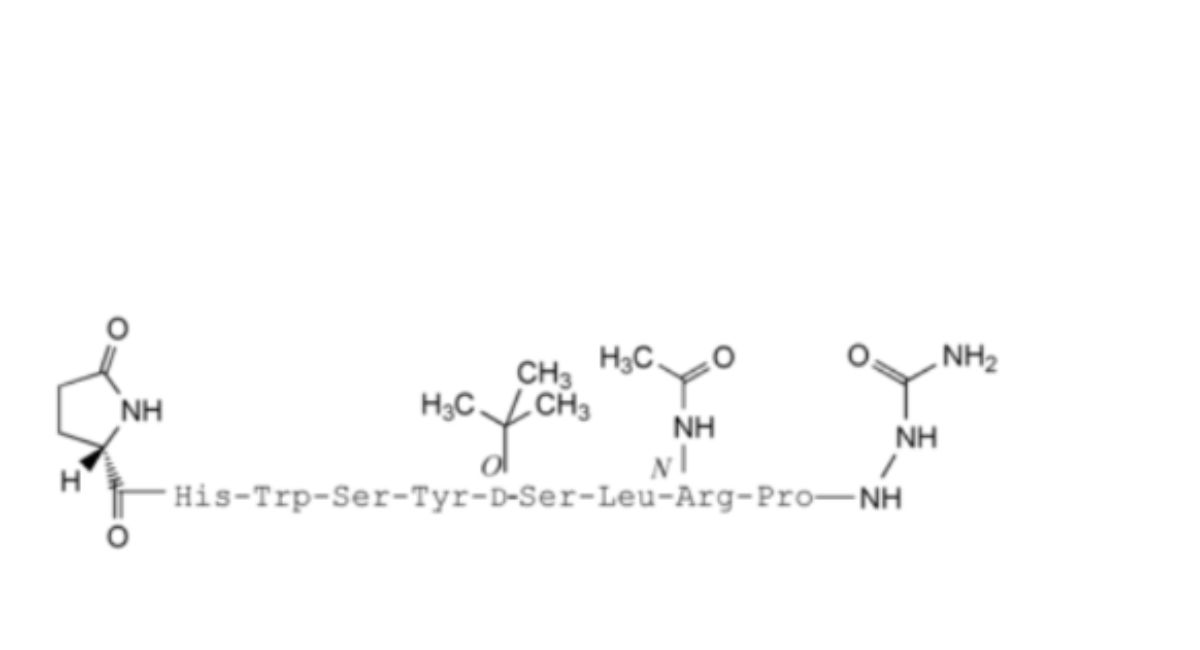
8. 8-(N-acetylamino-L-arginine)-goserelin,
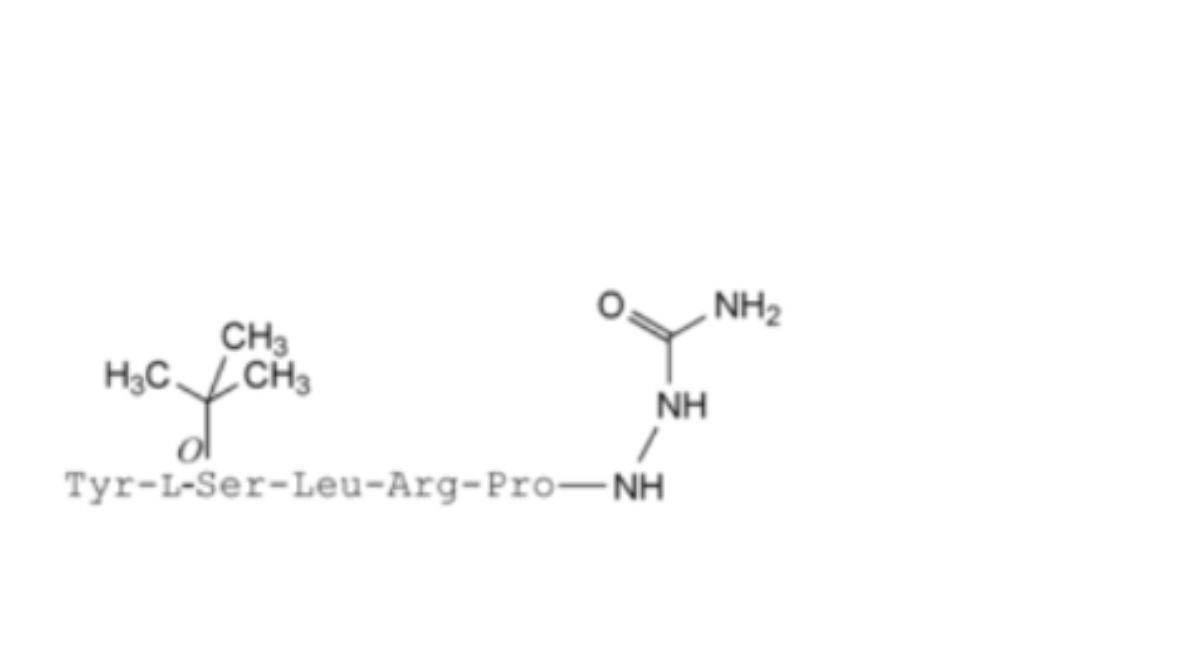
9. Hexapeptide,
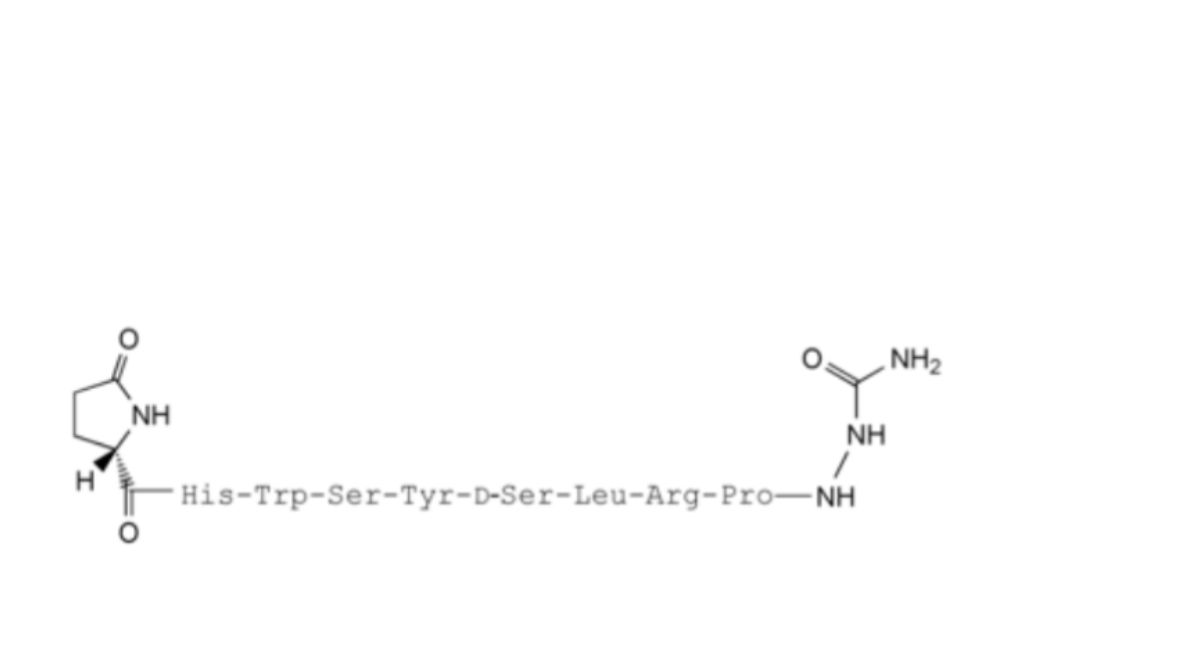
10. 6-(des-t-butyl-D-serine)-goserelin,
13. The structure of this peak has not been identified.