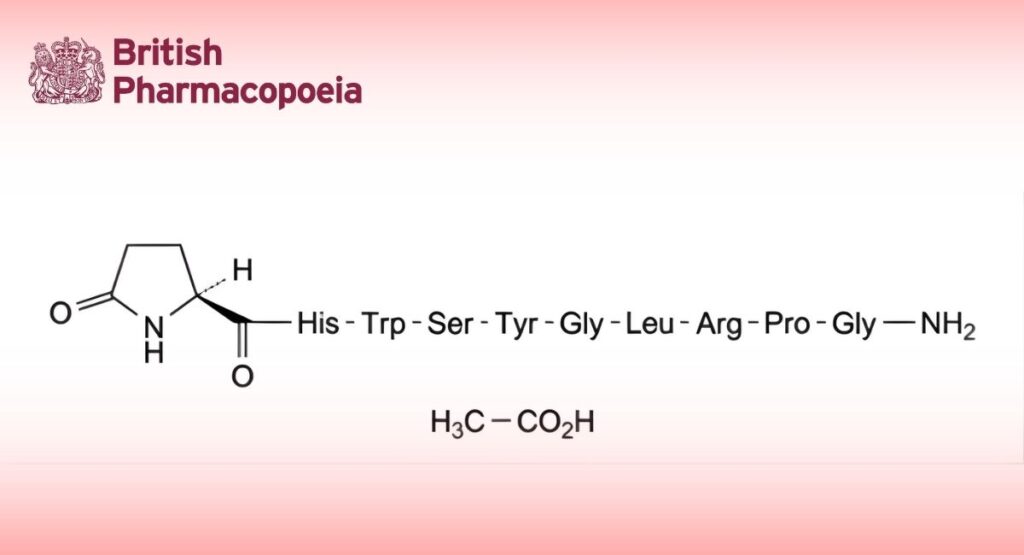
(Ph. Eur. monograph 0827)
C57H79N17O15 1242 499785-55-6
Action and use
Gonadotropin-releasing hormone; treatment of prostate cancer.
DEFINITION
5-Oxo-L-prolyl-L-histidyl-L-tryptophyl-L-seryl-L-tyrosylglycyl-L-leucyl-L-arginyl-L-prolylglycinamide acetate.
Acetate form of a synthetic hypothalamic peptide that stimulates the release of follicle-stimulating hormone and luteinising hormone from the pituitary gland.
Content
95.0 per cent to 102.0 per cent of the peptide C55H75N17O13 (anhydrous and acetic acid-free substance).
CHARACTERS
Appearance
White or slightly yellowish powder, hygroscopic.
Solubility
Soluble in water, sparingly soluble in methanol. It dissolves in a 1 per cent V/V solution of glacial acetic acid.
IDENTIFICATION
Carry out either tests A, B or tests A, C.
A. Examine the chromatograms obtained in the assay.
Results: The principal peak in the chromatogram obtained with the test solution is similar in retention time and size to the principal peak in the chromatogram obtained with reference solution (a).
B. Nuclear magnetic resonance spectrometry (2.2.64).
Preparation 4 mg/mL solution in a mixture of 20 volumes of deuterated acetic acid R and 80 volumes of deuterium oxide R.
Comparison: Dissolve the contents of a vial of gonadorelin for NMR identification CRS in a mixture of 20 volumes of deuterated acetic acid R and 80 volumes of deuterium oxide R to obtain a concentration of 4 mg/mL.
Operating conditions:
— field strength: minimum 300 MHz;
— temperature: 27 °C.
Results: Examine the H NMR spectrum from 0 ppm to 9 ppm. The H NMR spectrum obtained is qualitatively similar to the H NMR spectrum obtained with gonadorelin for NMR identification CRS.
C. Amino acid analysis (2.2.56). Method 1 for hydrolysis and method 1 for analysis are suitable.
Express the content of each amino acid in moles. Calculate the relative proportions of the amino acids, taking 1/8 of the sum of the number of moles of histidine, glutamic acid, leucine, proline, glycine, tyrosine and arginine as equal to 1. The values fall within the following limits: serine and tyrosine 0.7 to 1.05; glutamic acid, proline, leucine, histidine and arginine 0.9 to 1.1; glycine 1.8 to 2.2. Lysine and isoleucine are absent, and not more than traces of other amino acids are present.
TESTS
Specific optical rotation (2.2.7)
-65 to -58 (anhydrous and acetic acid-free substance).
Dissolve 10.0 mg in 1.0 mL of a 1 per cent V/V solution of glacial acetic acid R.
Related substances
Liquid chromatography (2.2.29).
Test solution: Dissolve the substance to be examined in water R to obtain a concentration of 1.0 mg/mL.
Reference solution (a): Dissolve the contents of a vial of gonadorelin CRS in water R to obtain a concentration of 1.0 mg/mL.
Reference solution (b): Dilute 1.0 mL of the test solution to 100.0 mL with water R.
Reference solution (c): Dissolve the contents of a vial of gonadorelin for system suitability A CRS (containing impurities C, E, F and G) in 1.0 mL of water R.
Column:
— size: l = 0.125 m, Ø = 4.0 mm;
— stationary phase: end-capped octadecylsilyl silica gel for chromatography R (5 μm).
Mobile phase: Mix 13 volumes of acetonitrile R1 and 87 volumes of a 1.18 per cent V/V solution of phosphoric acid R previously adjusted to pH 2.3 with triethylamine R.
Flow rate: 1.5 mL/min.
Detection: Spectrophotometer at 215 nm.
Injection: 10 μL of the test solution and reference solutions (b) and (c).
Run time: Twice the retention time of gonadorelin.
Relative retention: With reference to gonadorelin (retention time = about 12-16 min): impurity C = about 0.7; impurity E = about 0.8; impurities F and G = about 1.2.
System suitability: Reference solution (c):
— resolution: minimum 1.5 between the peaks due to impurities C and E.
Limits:
— impurity E: maximum 2.0 per cent;
— sum of impurities F and G: maximum 1.5 per cent;
— impurity C: maximum 1.0 per cent;
— unspecified impurities: for each impurity, maximum 0.5 per cent;
— total: maximum 5.0 per cent;
— reporting threshold: 0.1 per cent.
Acetic acid (2.5.34)
4.0 per cent to 7.5 per cent.
Test solution: Dissolve 10.0 mg of the substance to be examined in a mixture of 5 volumes of mobile phase B and 95 volumes of mobile phase A and dilute to 10.0 mL with the same mixture of solvents.
Water (2.5.32)
Maximum 7.0 per cent, determined on 20.0 mg.
ASSAY
Liquid chromatography (2.2.29) as described in the test for related substances with the following modification.
Injection: Test solution and reference solution (a).
Calculate the percentage content of gonadorelin (C55H75N17O13) taking into account the assigned content of C55H75N17O13 in gonadorelin CRS.
STORAGE
In an airtight container, protected from light at a temperature of 2 °C to 8 °C. If the substance is sterile, the container is also sterile and tamper-evident.
LABELLING
The label states:
— the gonadorelin peptide content;
— where applicable, that the substance is suitable for use in the manufacture of parenteral preparations.
IMPURITIES
Specified impurities C, E, F, G.
Other detectable impurities (the following substances would, if present at a sufficient level, be detected by one or other of the tests in the monograph. They are limited by the general acceptance criterion for other/unspecified impurities and/or by the general monograph Substances for pharmaceutical use (2034). It is therefore not necessary to identify these impurities for demonstration of compliance. See also 5.10. Control of impurities in substances for pharmaceutical use) D.

C. des-9-L-proline,10-L-glycine-gonadorelin,

D. [3-D-tryptophan]gonadorelin,

E. [2-D-histidine]gonadorelin,

F. [10-glycine]gonadorelin,

G. endo-5a-glycine-gonadorelin.