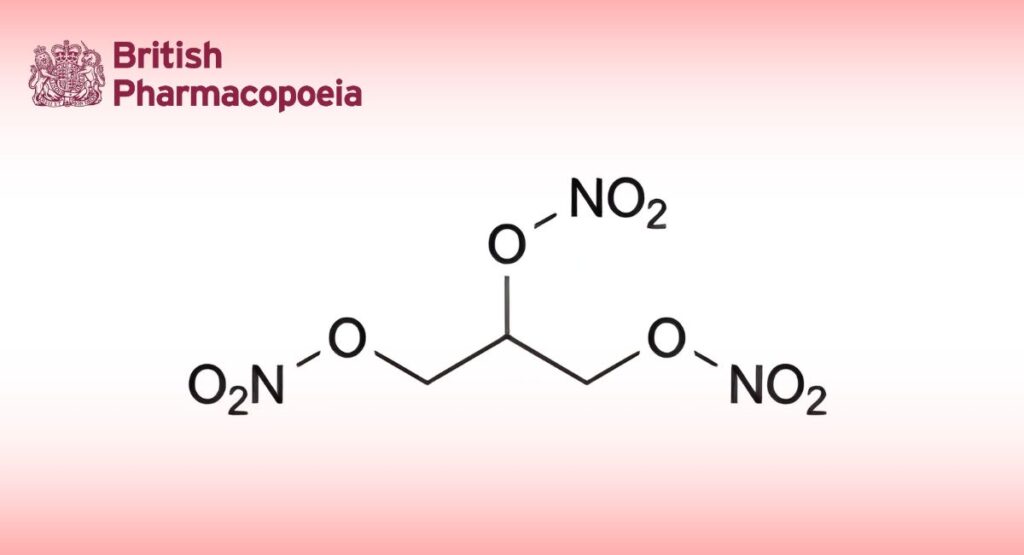
(Ph. Eur. monograph 1331)
C3H5N3O9 227.1
Action and use
Vasodilator.
Preparations
Glyceryl Trinitrate Ointment
Glyceryl Trinitrate Sublingual Spray
Glyceryl Trinitrate Tablets
Glyceryl Trinitrate Transdermal Patches
When concentrated glyceryl trinitrate solution is demanded, the intention of the purchaser, with respect to the strength expressed, should be ascertained.
DEFINITION
Ethanolic solution of glyceryl trinitrate containing 1 per cent m/m to 10 per cent m/m of propane-1,2,3-triyl trinitrate.
Content
96.5 per cent to 102.5 per cent of the declared content of glyceryl trinitrate stated on the label.
CHARACTERS
Appearance
Clear, colourless or slightly yellow solution.
Solubility
Miscible with acetone and with anhydrous ethanol.
Solubility of pure glyceryl trinitrate: practically insoluble in water, freely soluble in anhydrous ethanol, miscible with acetone.
IDENTIFICATION
First identification: A, C.
Second identification: B, C.
Upon diluting glyceryl trinitrate solution, care must be taken to always use anhydrous ethanol, otherwise droplets of pure glyceryl trinitrate may precipitate from the solution.
After examination, the residues and the solutions obtained in both the identification and the test sections must be heated on a water-bath for 5 min with dilute sodium hydroxide solution R.
A. Infrared absorption spectrophotometry (2.2.24).
Preparation: Place 50 μL of a solution diluted, if necessary, with anhydrous ethanol R, to contain 10 g/L of glyceryl trinitrate, on a disc of potassium bromide R and evaporate the solvent in vacuo.
Comparison Ph. Eur. reference spectrum of glyceryl trinitrate.
B. Thin-layer chromatography (2.2.27).
Test solution: Dilute a quantity of the substance to be examined corresponding to 50 mg of glyceryl trinitrate to 100 mL with acetone R.
Reference solution: Dilute 0.05 mL of glyceryl trinitrate solution CRS to 1 mL with acetone R.
Plate TLC silica gel plate R.
Mobile phase ethyl acetate R, toluene R (20:80 V/V).
Application 5 μL.
Development Over 2/3 of the plate.
Drying In air.
Detection: Spray with freshly prepared potassium iodide and starch solution R; expose to ultraviolet light at 254 nm for 15 min and examine in daylight.
Results The principal spot in the chromatogram obtained with the test solution is similar in position, colour and size to the principal spot in the chromatogram obtained with the reference solution.
C. It complies with the limits of the assay.
TESTS
Upon diluting glyceryl trinitrate solution, care must be taken to always use anhydrous ethanol, otherwise droplets of pure glyceryl trinitrate may precipitate from the solution.
After examination, the residues and the solutions obtained in both the identification and the test sections must be heated on a water-bath for 5 min with dilute sodium hydroxide solution R.
Appearance of solution
If necessary, dilute the solution to be examined to a concentration of 10 g/L with anhydrous ethanol R. The solution is not more intensely coloured than reference solution Y7 (2.2.2, Method II).
Impurity A (inorganic nitrates)
Thin-layer chromatography (2.2.27).
Test solution If necessary, dilute the solution to be examined to a concentration of 10 g/L with anhydrous ethanol R.
Reference solution: Dissolve 5 mg of potassium nitrate R in 1 mL of water R and dilute to 100 mL with ethanol (96 per cent) R.
Plate TLC silica gel plate R.
Mobile phase glacial acetic acid R, acetone R, toluene R (15:30:60 V/V/V).
Application 10 μL.
Development: Over 2/3 of the plate.
Drying: In a current of air until the acetic acid is completely removed.
Detection: Spray intensively with freshly prepared potassium iodide and starch solution R; expose to ultraviolet light at 254 nm for 15 min and examine in daylight.
Limit:
— nitrate ion: any spot due to the nitrate ion in the chromatogram obtained with the test solution is not more intense than the spot in the chromatogram obtained with the reference solution (0.5 per cent of the content of glyceryl trinitrate calculated as potassium nitrate).
Related substances
Liquid chromatography (2.2.29).
Test solution: Dilute a quantity of the substance to be examined equivalent to 5.0 mg of glyceryl trinitrate to 10.0 mL with water for chromatography R. NOTE: for glyceryl trinitrate solutions of more than 1 per cent m/m, dilute first to a 1 per cent solution with anhydrous ethanol R.
Reference solution (a): Dilute 1.0 mL of the test solution to 100.0 mL with water for chromatography R.
Reference solution (b): Dilute 25 μL of glyceryl trinitrate for system suitability CRS (containing impurities B, C, D and E) to 0.5 mL with water for chromatography R.
Column:
— size: l = 0.25 m, Ø = 4.6 mm;
— stationary phase: end-capped octadecylsilyl silica gel for chromatography R (5 μm);
— temperature: 40 °C.
Mobile phase:
— mobile phase A: water for chromatography R;
— mobile phase B: acetonitrile R1;
| Time (min) |
Mobile phase A (per cent V/V) |
Mobile phase B (per cent V/V) |
| 0 – 2 | 90 | 10 |
| 2 – 10 | 90 → 50 | 10 → 50 |
| 10 – 20 | 50 | 50 |
Flow rate 1.5 mL/min.
Detection: Spectrophotometer at 210 nm.
Injection 20 μL.
Identification of impurities: Use the chromatogram supplied with glyceryl trinitrate for system suitability CRS and the chromatogram obtained with reference solution (b) to identify the peaks due to impurities B, C, D and E.
Relative retention: With reference to glyceryl trinitrate (retention time = about 15.5 min): impurity C = about 0.19; impurity B = about 0.21; impurity E = about 0.63; impurity D = about 0.65.
System suitability Reference solution (b):
— resolution: minimum 1.5 between the peaks due to impurities C and B; minimum 1.5 between the peaks due to impurities E and D.
Calculation of percentage contents:
— for each impurity, use the concentration of glyceryl trinitrate in reference solution (a).
Limits:
— impurities B, C, D, E: for each impurity, maximum 0.5 per cent;
— unspecified impurities: for each impurity, maximum 0.10 per cent;
— total: maximum 1.5 per cent;
— reporting threshold: 0.05 per cent.
ASSAY
Test solution: Prepare a solution containing 1.0 mg of glyceryl trinitrate in 250.0 mL of methanol R.
Reference solution: Dissolve 70.0 mg of sodium nitrite R in methanol R and dilute to 250.0 mL with the same solvent.
Dilute 5.0 mL of the solution to 500.0 mL with methanol R.
Into three 50 mL volumetric flasks introduce 10.0 mL of the test solution, 10.0 mL of the reference solution and 10 mL of methanol R as a blank. To each flask add 5 mL of dilute sodium hydroxide solution R, close the flask, mix and allow to stand at room temperature for 30 min. Add 10 mL of sulfanilic acid solution R and 10 mL of dilute hydrochloric acid R and mix. After exactly 4 min, add 10 mL of naphthylethylenediamine dihydrochloride solution R, dilute to volume with water R and mix. After 10 min read the absorbance (2.2.25) of the test solution and the reference solution at 540 nm using the blank solution as the compensation liquid.
Calculate the percentage content of glyceryl trinitrate using the following expression:
ATxmsxC/ARxmTx60.8
AT = absorption of the test solution;
mS = mass of sodium nitrite, in milligrams.
C = percentage content of sodium nitrite used as reference;
AR = absorption of the reference solution;
mT = mass of the substance to be examined, in milligrams;
STORAGE
Store the diluted solutions (1 per cent m/m) protected from light, at a temperature of 2 °C to 15 °C.
Store the other solutions protected from light, at a temperature of 15 °C to 20 °C.
LABELLING
The label states the declared content of glyceryl trinitrate.
IMPURITIES
Specified impurities A, B, C, D, E.
A. inorganic nitrates,
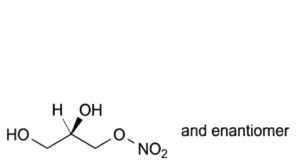
B. (2RS)-2,3-dihydroxypropyl nitrate,
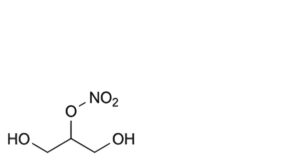
C. 2-hydroxy-1-(hydroxymethyl)ethyl nitrate,
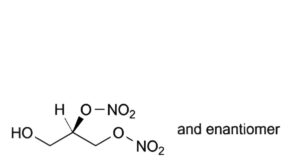
D. (2RS)-3-hydroxypropane-1,2-diyl dinitrate,
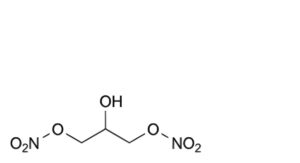
E. 2-hydroxypropane-1,3-diyl dinitrate.