(Ph. Eur. monograph 0495)
Action and use
Excipient.
DEFINITION
Mixture of monoacylglycerols, mainly monostearoylglycerol, together with variable quantities of di- and triacylglycerols. It is obtained by partial glycerolysis of vegetable oils mainly containing triacylglycerols of palmitic (hexadecanoic) or stearic (octadecanoic) acid or by esterification of glycerol with stearic acid. The fatty acids may be of vegetable or animal origin.
Content
— monoacylglycerols: 40.0 per cent to 55.0 per cent;
— diacylglycerols: 30.0 per cent to 45.0 per cent;
— triacylglycerols: 5.0 per cent to 15.0 per cent.
CHARACTERS
Appearance
Hard, waxy mass or unctuous powder or flakes, white or almost white.
Solubility
Practically insoluble in water, soluble in ethanol (96 per cent) at 60 °C.
IDENTIFICATION
First identification: C, D.
Second identification: A, B.
A. Melting point (2.2.15): 54 °C to 66 °C.
Introduce the melted substance into the capillary tubes and allow to stand for 24 h in a well-closed container.
B. Thin-layer chromatography (2.2.27).
Test solution: Dissolve 0.5 g of the substance to be examined in methylene chloride R, with gentle heating, and dilute to 10 mL with the same solvent.
Reference solution: Dissolve 0.5 g of glycerol monostearate 40-55 CRS in methylene chloride R, with gentle heating, and dilute to 10 mL with the same solvent.
Plate: TLC silica gel plate R.
Mobile phase: hexane R, ether R (30:70 V/V).
Application: 10 μL.
Development: Over a path of 15 cm.
Detection: Spray with a 0.1 g/L solution of rhodamine B R in ethanol (96 per cent) R and examine in ultraviolet light at 365 nm.
Suitability system: Reference solution:
— the chromatogram shows 4 clearly separated spots.
Results: The spots in the chromatogram obtained with the test solution are similar in position to those in the chromatogram obtained with the reference solution.
C. Composition of fatty acids (see Tests) according to the type stated on the label.
D. It complies with the limits of the assay (monoacylglycerol content).
TESTS
Acid value (2.5.1)
Maximum 3.0, determined on 1.0 g.
Use a mixture of equal volumes of ethanol (96 per cent) R and toluene R as solvent and heat gently.
Iodine value (2.5.4, Method A)
Maximum 3.0.
Saponification value (2.5.6)
158 to 177, determined on 2.0 g. Carry out the titration with heating.
Free glycerol
Maximum 6.0 per cent, determined as described under Assay.
Composition of fatty acids (2.4.22, Method C)
Use the mixture of calibrating substances in Table 2.4.22.-1.
Composition of the fatty-acid fraction of the substance:
| Glycerol monostearate 40-55 | Composition of fatty acids |
| Type I | Stearic acid: 40.0 per cent to 60.0 per cent
Sum of the contents of palmitic and stearic acids: minimum 90.0 per cent |
| Type II | Stearic acid: 60.0 per cent to 80.0 per cent
Sum of the contents of palmitic and stearic acids: minimum 90.0 per cent |
| Type III | Stearic acid: 80.0 per cent to 99.0 per cent
Sum of the contents of palmitic and stearic acids: minimum 96.0 per cent |
Water (2.5.12)
Maximum 1.0 per cent, determined on 1.00 g. Use pyridine R as the solvent and heat gently.
Total ash (2.4.16)
Maximum 0.1 per cent.
ASSAY
Size-exclusion chromatography (2.2.30).
Test solution: Into a 15 mL flask, weigh 0.200 g (m). Add 5.0 mL of tetrahydrofuran R and shake to dissolve. Reweigh the flask and calculate the total mass of solvent and substance (M).
Reference solutions: Into four 15 mL flasks, respectively weigh 2.5 mg, 5.0 mg, 10.0 mg and 20.0 mg of glycerol R, and add 5.0 mL of tetrahydrofuran R to each flask. Weigh the flasks again and calculate the concentration of glycerol in milligrams per gram for each reference solution.
Column:
— size: l = 0.6 m, Ø = 7 mm;
— stationary phase: styrene-divinylbenzene copolymer R (5 μm) with a pore size of 10 nm.
Mobile phase: tetrahydrofuran R.
Flow rate: 1 mL/min.
Detection: Differential refractometer.
Injection 40 μL.
Relative retention: With reference to glycerol (retention time = about 15 min): triacylglycerols = about 0.75; diacylglycerols = about 0.80; monoacylglycerols = about 0.85.
Calculations:
— free glycerol: from the calibration curve obtained with the reference solutions, determine the concentration of glycerol (C) in milligrams per gram in the test solution and calculate the percentage content of free glycerol (A) in the substance to be examined using the following expression:
C x M/m x 10
— free fatty acids: calculate the percentage content of free fatty acids (D) using the following expression:
IA x 270/56.11 x 10
IA = acid value (see Tests);
270 = average rounded molar mass of stearic acid and palmitic acid, in grams per mole;
56.11 = molar mass of potassium hydroxide, in grams per mole;
— monoacylglycerols: calculate the percentage content of monoacylglycerols using the following expression:
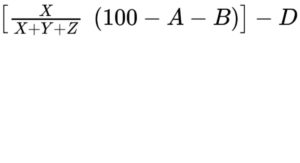
A = percentage content of free glycerol;
B = percentage content of water (see Tests);
D = percentage content of free fatty acids;
X = area of the peak due to monoacylglycerols and free fatty acids;
Y = area of the peak due to diacylglycerols;
Z = area of the peak due to triacylglycerols;
— diacylglycerols: calculate the percentage content of diacylglycerols using the following expression:
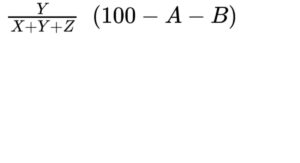
— triacylglycerols: calculate the percentage content of triacylglycerols using the following expression:
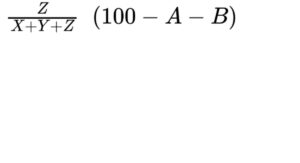
LABELLING
The label states the type of glycerol monostearate 40-55.
FUNCTIONALITY-RELATED CHARACTERISTICS
This section provides information on characteristics that are recognised as being relevant control parameters for one or more functions of the substance when used as an excipient (see chapter 5.15). Some of the characteristics described in the Functionality-related characteristics section may also be present in the mandatory part of the monograph since they also represent mandatory quality criteria. In such cases, a cross-reference to the tests described in the mandatory part is included in the
Functionality-related characteristics section. Control of the characteristics can contribute to the quality of a medicinal product by improving the consistency of the manufacturing process and the performance of the medicinal product during use. Where control methods are cited, they are recognised as being suitable for the purpose, but other methods can also be used. Wherever results for a particular characteristic are reported, the control method must be indicated.
The following characteristics may be relevant for glycerol monostearate 40-55 used as matrix former in prolonged-release oral solid dosage forms.
Composition of fatty acids
(see Tests).
Particle-size distribution (2.9.31 or 2.9.38)
Powder flow (2.9.36)
Thermal analysis (2.2.34)
The melting behaviour of the substance as is and after melting followed by solidification when cooling may be considered.
The following characteristics may be relevant for glycerol monostearate 40-55 used as consistency agent in dosage forms for cutaneous application.
Composition of fatty acids
(see Tests).
Thermal analysis (2.2.34)
The melting behaviour of the substance as is and after melting followed by solidification when cooling may be considered.






