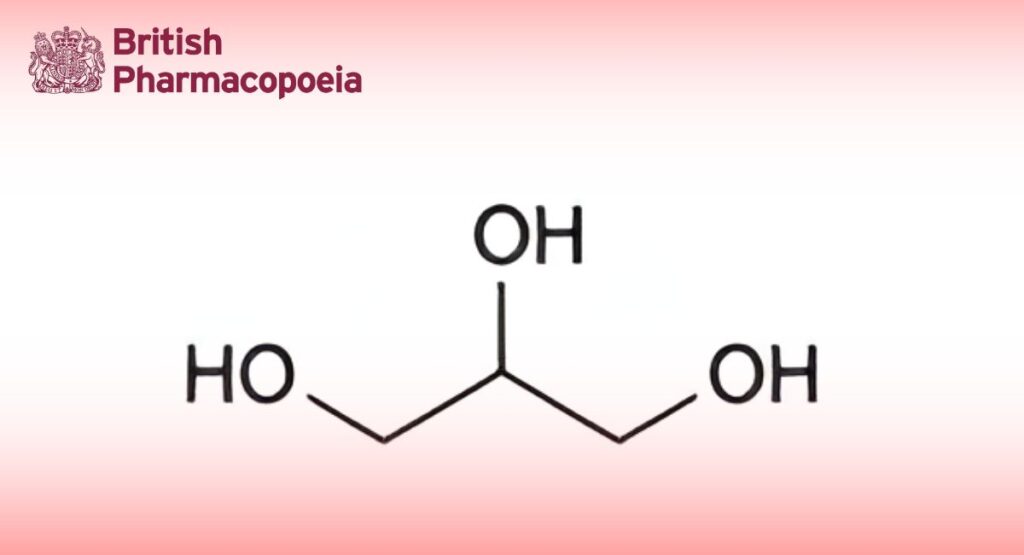
(Ph. Eur. Monograph 0496)
C3H8O3 92.1 56-81-5
Action and use
Lubricant; laxative.
Preparations
Glycerol Eye Drops
Glycerol Suppositories
DEFINITION
Propane-1,2,3-triol.
Content
98.0 per cent m/m to 101.0 per cent m/m (anhydrous substance).
CHARACTERS
Appearance
Clear, colourless or almost colourless, very hygroscopic, syrupy liquid, unctuous to the touch.
Solubility
Miscible with water and with ethanol (96 per cent), slightly soluble in acetone, practically insoluble in fatty oils and in essential oils.
IDENTIFICATION
First identification: A, B.
Second identification: A, C.
A. Refractive index (see Tests).
B. Infrared absorption spectrophotometry (2.2.24).
Preparation: To 5 mL add 1 mL of water R and mix carefully.
Comparison: Ph. Eur. reference spectrum of glycerol (85 per cent).
C. Relative density (2.2.5): 1.258 to 1.268.
TESTS
Solution S
Dilute 100.0 g to 200.0 mL with carbon dioxide-free water R.
Appearance of solution
Solution S is clear (2.2.1). Dilute 10 mL of solution S to 25 mL with water R. The solution is colourless (2.2.2, Method II).
Acidity or alkalinity
To 50 mL of solution S add 0.5 mL of phenolphthalein solution R. The solution is colourless. Not more than 0.2 mL of 0.1 M sodium hydroxide is required to change the colour of the indicator to pink.
Refractive index (2.2.6)
1.470 to 1.475.
Aldehydes
Maximum 10 ppm.
Place 7.5 mL of solution S in a ground-glass-stoppered flask and add 7.5 mL of water R and 1.0 mL of decolorised pararosaniline solution R. Close the flask and allow to stand for 1 h at a temperature of 25 ± 1 °C. The absorbance (2.2.25) of the solution measured at 552 nm is not greater than that of a standard prepared at the same time and in the same
manner using 7.5 mL of formaldehyde standard solution (5 ppm CH2O) R and 7.5 mL of water R. The test is not valid unless the standard is pink.
Esters
Add 10.0 mL of 0.1 M sodium hydroxide to the final solution obtained in the test for acidity or alkalinity. Boil under a reflux condenser for 5 min. Cool. Add 0.5 mL of phenolphthalein solution R and titrate with 0.1 M hydrochloric acid. Not less than 8.0 mL of 0.1 M hydrochloric acid is required to change the colour of the indicator.
Impurity A and related substances
Gas chromatography (2.2.28).
Test solution: Dilute 10.0 mL of solution S to 100.0 mL with water R.
Reference solution (a): Dilute 10.0 g of glycerol R1 to 20.0 mL with water R. Dilute 10.0 mL of the solution to 100.0 mL with water R.
Reference solution (b): Dissolve 1.000 g of diethylene glycol R in water R and dilute to 100.0 mL with the same solvent.
Reference solution (c): Dilute 1.0 mL of reference solution (b) to 10.0 mL with reference solution (a). Dilute 1.0 mL of this solution to 20.0 mL with reference solution (a).
Reference solution (d): Mix 1.0 mL of the test solution and 5.0 mL of reference solution (b) and dilute to 100.0 mL with water R. Dilute 1.0 mL of this solution to 10.0 mL with water R.
Reference solution (e): Dilute 5.0 mL of reference solution (b) to 100.0 mL with water R.
Column:
— size: l = 30 m, Ø = 0.53 mm;
— stationary phase: cyanopropyl(3)phenyl(3)methyl(94)polysiloxane R.
Carrier gas helium for chromatography R.
Split ratio 1:10.
Linear velocity 38 cm/s.
Temperature:
| Time (min) |
Temperature (°C) |
|
| Column | 0 | 100 |
| 0 – 16 | 100 → 220 | |
| 16 – 20 | 220 | |
| Injection port | 220 | |
| Detector | 250 |
Detection: Flame ionisation.
Injection: 0.5 μL.
Elution order: Impurity A, glycerol.
System suitability: Reference solution (d):
— resolution: minimum 7.0 between the peaks due to impurity A and glycerol.
Limits:
— impurity A: not more than the area of the corresponding peak in the chromatogram obtained with reference solution (c) (0.1 per cent);
— any other impurity with a retention time less than the retention time of glycerol: not more than the area of the peak due to impurity A in the chromatogram obtained with reference solution (c) (0.1 per cent);
— total of all impurities with retention times greater than the retention time of glycerol: not more than 5 times the area of the peak due to impurity A in the chromatogram obtained with reference solution (c) (0.5 per cent);
— disregard limit: 0.05 times the area of the peak due to impurity A in the chromatogram obtained with reference solution (e) (0.05 per cent).
Halogenated compounds
Maximum 35 ppm.
To 10 mL of solution S add 1 mL of dilute sodium hydroxide solution R, 5 mL of water R and 50 mg of halogen-free nickel- aluminium alloy R. Heat on a water-bath for 10 min, allow to cool and filter. Rinse the flask and the filter with water R until 25 mL of filtrate is obtained. To 5 mL of the filtrate add 4 mL of ethanol (96 per cent) R, 2.5 mL of water R, 0.5 mL of nitric acid R and 0.05 mL of silver nitrate solution R2 and mix. Allow to stand for 2 min. Any opalescence in the solution is not more intense than that in a standard prepared at the same time by mixing 7.0 mL of chloride standard solution (5 ppm Cl) R, 4 mL of ethanol (96 per cent) R, 0.5 mL of water R, 0.5 mL of nitric acid R and 0.05 mL of silver nitrate solution R2.
Sugars
To 10 mL of solution S add 1 mL of dilute sulfuric acid R and heat on a water-bath for 5 min. Add 3 mL of an 85 mg/mL solution of sodium hydroxide R in carbon dioxide-free water R, mix and add dropwise 1 mL of freshly prepared copper sulfate solution R. The solution is clear and blue. Continue heating on the water-bath for 5 min. The solution remains blue
and no precipitate is formed.
Chlorides (2.4.4)
Maximum 10 ppm.
Dilute 1 mL of solution S to 15 mL with water R. Prepare the standard using 1 mL of chloride standard solution (5 ppm Cl) R diluted to 15 mL with water R.
Water (2.5.12)
Maximum 2.0 per cent, determined on 1.000 g.
Sulfated ash (2.4.14)
Maximum 0.01 per cent, determined on 5.0 g after heating to boiling and ignition.
ASSAY
Thoroughly mix 0.075 g with 45 mL of water R. Add 25.0 mL of a mixture of 1 volume of 0.1 M sulfuric acid and 20 volumes of 0.1 M sodium periodate. Allow to stand protected from light for 15 min. Add 5.0 mL of a 500 g/L solution of ethylene glycol R and allow to stand protected from light for 20 min. Using 0.5 mL of phenolphthalein solution R as indicator, titrate with 0.1 M sodium hydroxide. Carry out a blank titration.
1 mL of 0.1 M sodium hydroxide is equivalent to 9.21 mg of C3H8O3.
STORAGE
In an airtight container.
IMPURITIES
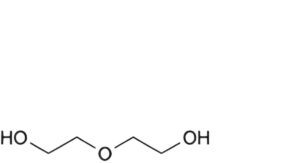
A. 2,2′-oxydi(ethan-1-ol) (diethylene glycol),
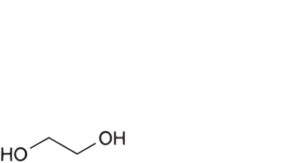
B. ethane-1,2-diol (ethylene glycol),
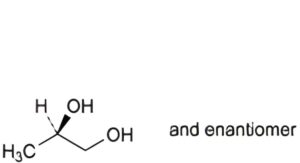
C. (2RS)-propane-1,2-diol (propylene glycol).