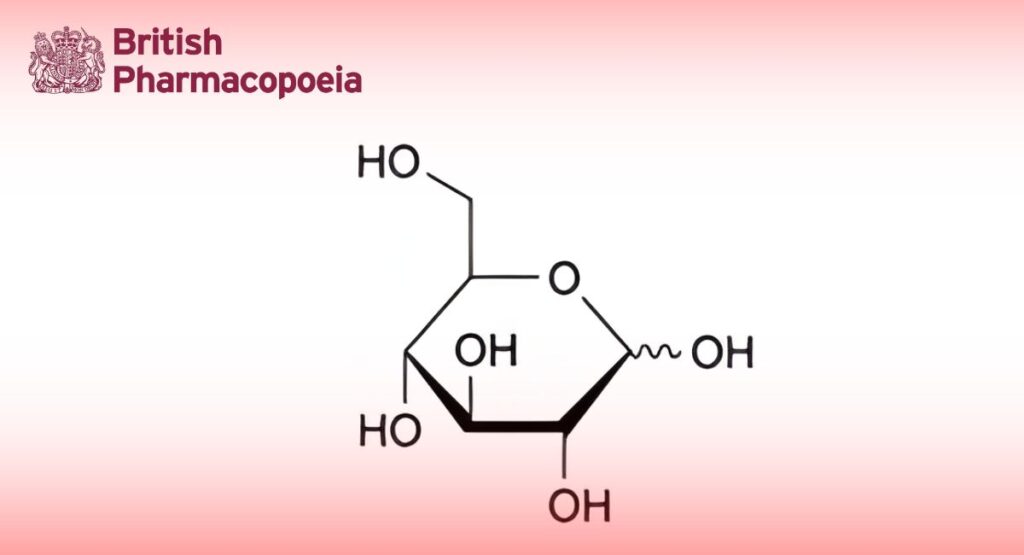
Anhydrous Glucose
(Ph. Eur. monograph 0177)
C6H12O6 180.2 50-99-7
Preparations
Glucose Infusion
Compound Glucose, Sodium Chloride and Sodium Citrate Oral Solution
Oral Rehydration Salts
Potassium Chloride and Glucose Intravenous Infusion
Potassium Chloride, Sodium Chloride and Glucose Intravenous Infusion
Sodium Chloride and Glucose Intravenous Infusion
DEFINITION
D-Glucopyranose.
It is derived from starch.
Content
97.5 per cent to 102.0 per cent (anhydrous substance).
♦ CHARACTERS
Appearance
White or almost white, crystalline powder.
Solubility
Freely soluble in water, very slightly soluble in ethanol (96 per cent).♦
IDENTIFICATION
First identification: ♢ A♢ , B, E.
♢ Second identification: C, D.♢
♢ A. Specific optical rotation (2.2.7): + 52.5 to + 53.3 (anhydrous substance).
Dissolve 10.0 g in 80 mL of water R, add 0.2 mL of dilute ammonia R1, allow to stand for 30 min and dilute to 100.0 mL with water R.♢
B. Examine the chromatograms obtained in the assay.
Results: The principal peak in the chromatogram obtained with the test solution is similar in retention time and size to the principal peak in the chromatogram obtained with reference solution (a).
♢ C. Thin-layer chromatography (2.2.27).
Solvent mixture: water R, methanol R (40:60 V/V).
Test solution: Dissolve 10 mg of the substance to be examined in the solvent mixture and dilute to 20 mL with the solvent mixture.
Reference solution: Dissolve 10 mg of glucose monohydrate CRS in the solvent mixture and dilute to 20 mL with the solvent mixture.
Plate: TLC silica gel plate R.
Mobile phase: water R, methanol R, anhydrous acetic acid R, methylene chloride R (10:15:25:50 V/V/V/V); measure the volumes accurately since a slight excess of water produces cloudiness.
Application: 2 μL; thoroughly dry the points of application.
Development A: Over 3/4 of the plate.
Drying A: In a current of warm air.
Development B: Immediately, over 3/4 of the plate, after renewing the mobile phase.
Drying B: In a current of warm air.
Detection: Treat with a solution of 0.5 g of thymol R in a mixture of 5 mL of sulfuric acid R and 95 mL of ethanol (96 per cent) R; heat at 130 °C for 10 min.
Results: The principal spot in the chromatogram obtained with the test solution is similar in position, colour and size to the principal spot in the chromatogram obtained with the reference solution.
D. Dissolve 5 g in water R and dilute to 10 mL with the same solvent. To 0.5 mL of the solution, add 0.2 g of resorcinol R and 9 mL of dilute hydrochloric acid R and heat on a water-bath for 2 min. No red colour develops.♢
E. Water (see Tests).
TESTS
Appearance of solution
The solution is clear (2.2.1) and not more intensely coloured than reference solution BY7 (2.2.2, Method II).
Dissolve 10.0 g in 15 mL of water R, heating on a water-bath.
Conductivity (2.2.38)
Maximum 20 μS·cm .
Dissolve 20.0 g in carbon dioxide-free water R prepared from distilled water R and dilute to 100.0 mL with the same solvent. Measure the conductivity of the solution while gently stirring with a magnetic stirrer.
Related substances
Liquid chromatography (2.2.29).
Test solution: Dissolve 0.300 g of the substance to be examined in water R and dilute to 10.0 mL with the same solvent.
Reference solution (a): Dissolve 0.330 g of glucose monohydrate CRS in water R and dilute to 10.0 mL with the same solvent.
Reference solution (b): Dilute 1.0 mL of the test solution to 250.0 mL with water R.
Reference solution (c): Dilute 25.0 mL of reference solution (b) to 200.0 mL with water R.
Reference solution (d): Dissolve 5 mg of fructose R (impurity D), 5 mg of maltose monohydrate R (impurity A) and 5 mg of maltotriose R (impurity C) in water R and dilute to 50 mL with the same solvent.
Column:
— size: l = 0.3 m, Ø = 7.8 mm;
— stationary phase: strong cation-exchange resin (calcium form) R (9 μm);
— temperature: 85 ± 1 °C.
Mobile phase: Degassed water for chromatography R.
Flow rate: 0.3 mL/min.
Detection: Refractometer maintained at a constant temperature (40 °C for example).
Injection: 20 μL of the test solution and reference solutions (b), (c) and (d).
Run time: 1.5 times the retention time of glucose.
Relative retention: With reference to glucose (retention time = about 21 min): impurity C = about 0.7; impurities A and B = about 0.8; impurity D = about 1.3.
System suitability: Reference solution (d):
— resolution: minimum 1.3 between the peaks due to impurities C and A.
Limits:
— sum of impurities A and B: not more than the area of the principal peak in the chromatogram obtained with reference solution (b) (0.4 per cent);
— impurity C: not more than 0.5 times the area of the principal peak in the chromatogram obtained with reference solution (b) (0.2 per cent);
— impurity D: not more than 3 times the area of the principal peak in the chromatogram obtained with reference solution (c) (0.15 per cent);
— unspecified impurities: for each impurity, not more than twice the area of the principal peak in the chromatogram obtained with reference solution (c) (0.10 per cent);
— total: not more than 1.25 times the area of the principal peak in the chromatogram obtained with reference solution (b) (0.5 per cent);
— disregard limit: the area of the principal peak in the chromatogram obtained with reference solution (c) (0.05 per cent).
The thresholds indicated under Related substances (Table 2034.-1) in the general monograph Substances for pharmaceutical use (2034) do not apply.
Dextrin
To 1 g of the finely powdered substance to be examined add 20 mL of ethanol (96 per cent) R and heat under a reflux condenser. The substance dissolves completely.
Soluble starch, sulfite
Maximum 15 ppm.
Dissolve 6.7 g in 15.0 mL of water R, heating on a water-bath. Allow to cool and add 25 μL of iodine solution R5. The solution is yellow.
Water (2.5.12)
Maximum 1.0 per cent, determined on 0.500 g.
♢ Pyrogens (2.6.8)
If intended for use in the manufacture of large-volume parental preparations without a further appropriate procedure for the removal of pyrogens, the competent authority may require that it comply with the test for pyrogens. Inject per kilogram of the rabbit’s mass 10 mL of a solution in water for injections R containing 50 mg of the substance to be examined per millilitre.♢
ASSAY
Liquid chromatography (2.2.29) as described in the test for related substances with the following modification.
Injection: Test solution and reference solution (a).
Calculate the percentage content of C6H12O6 taking into account the assigned content of glucose monohydrate CRS.
IMPURITIES
Specified impurities A, B, C, D.
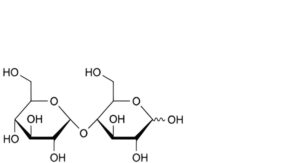
A. 4-O-α-D-glucopyranosyl-D-glucopyranose (maltose),
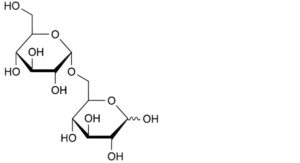
B. 6-O-α-D-glucopyranosyl-D-glucopyranose (isomaltose),
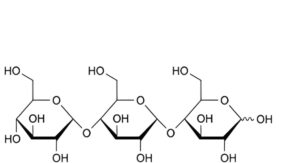
C. α-D-glucopyranosyl-(1→4)-α-D-glucopyranosyl-(1→4)-D-glucopyranose (maltotriose),
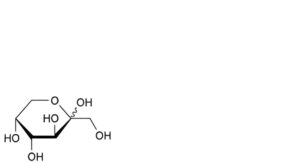
D. D-arabino-hex-2-ulopyranose (fructose).
This monograph has undergone pharmacopoeial harmonisation. See chapter 5.8 Pharmacopoeial harmonisation.