(Ph. Eur. monograph 0718)
C23H28ClN3O5S 494.0 10238-21-8
Action and use
Inhibition of ATP-dependent potassium channels (sulfonylurea); treatment of diabetes mellitus.
Preparation
Glibenclamide Tablets
DEFINITION
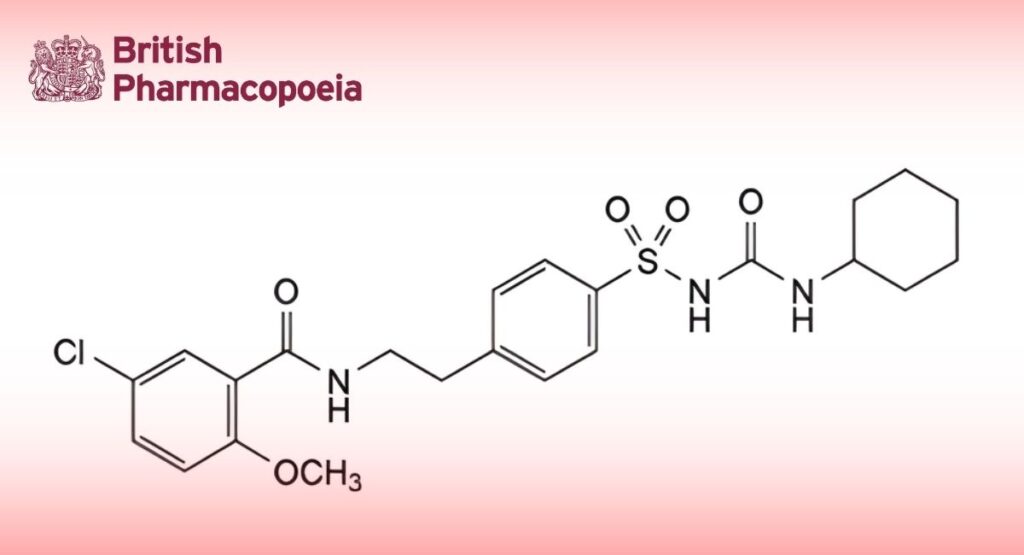
1-[[4-[2-[(5-Chloro-2-methoxybenzoyl)amino]ethyl]phenyl]sulfonyl]-3-cyclohexylurea.
Content
99.0 per cent to 101.0 per cent (dried substance).
CHARACTERS
Appearance
White or almost white, crystalline powder.
Solubility
Practically insoluble in water, sparingly soluble in methylene chloride, slightly soluble in ethanol (96 per cent) and in methanol.
It shows polymorphism (5.9).
IDENTIFICATION
First identification: C.
Second identification: A, B, D, E.
A. Melting point (2.2.14): 169 °C to 174 °C.
B. Ultraviolet and visible absorption spectrophotometry (2.2.25).
Test solution: Dissolve 50.0 mg in methanol R, with the aid of ultrasound if necessary, and dilute to 50.0 mL with the same solvent. To 10.0 mL of the solution add 1.0 mL of a 103 g/L solution of hydrochloric acid R and dilute to 100.0 mL with methanol R.
Spectral range: 230-350 nm.
Absorption maxima: At 300 nm and a less intense maximum at 275 nm.
Specific absorbance at the absorption maxima:
— at 300 nm: 61 to 65;
— at 275 nm: 27 to 32.
C. Infrared absorption spectrophotometry (2.2.24).
Comparison glibenclamide CRS.
If the spectra obtained show differences, moisten separately the substance to be examined and the reference substance with methanol R, triturate, dry at 100-105 °C and record the spectra again.
D. Thin-layer chromatography (2.2.27).
Test solution: Dissolve 10 mg of the substance to be examined in a mixture of equal volumes of methanol R and methylene chloride R and dilute to 10 mL with the same mixture of solvents.
Reference solution: Dissolve 10 mg of glibenclamide CRS in a mixture of equal volumes of methanol R and methylene chloride R and dilute to 10 mL with the same mixture of solvents.
Plate: TLC silica gel GF254 plate R.
Mobile phase: ethanol (96 per cent) R, glacial acetic acid R, cyclohexane R, methylene chloride R (5:5:45:45 V/V/V/V).
Application: 10 μL.
Development:: Over 1/2 of the plate.
Drying: In air.
Detection: Examine in ultraviolet light at 254 nm.
Results: The principal spot in the chromatogram obtained with the test solution is similar in position and size to the principal spot in the chromatogram obtained with the reference solution.
E. Dissolve 20 mg in 2 mL of sulfuric acid R. The solution is colourless and shows blue fluorescence in ultraviolet light at 365 nm. Dissolve 0.1 g of chloral hydrate R in the solution. After about 5 min, the colour changes to deep yellow and, after about 20 min, develops a brownish tinge.
TESTS
Related substances
Liquid chromatography (2.2.29). Prepare the solutions immediately before use or store them at 5 °C for not more than 40 h.
Test solution: Dissolve 25.0 mg of the substance to be examined in methanol R, with the aid of ultrasound if necessary, and dilute to 10.0 mL with the same solvent.
Reference solution (a): Dissolve 3.0 mg of glibenclamide impurity A CRS and 3 mg of glibenclamide impurity B CRS in methanol R and dilute to 100.0 mL with the same solvent. Dilute 5.0 mL of the solution to 20.0 mL with methanol R.
Reference solution (b): Dilute 1.0 mL of the test solution to 100.0 mL with methanol R. Dilute 1.0 mL of this solution to 10.0 mL with methanol R.
Reference solution (c): Dissolve 12.5 mg of glibenclamide for peak identification CRS (containing impurity C) in methanol R, with the aid of ultrasound if necessary, and dilute to 5.0 mL with the same solvent.
Column:
— size: l = 0.10 m, Ø = 4.6 mm;
— stationary phase: spherical base-deactivated end-capped octadecylsilyl silica gel for chromatography R (3 μm);
— temperature: 35 °C.
Mobile phase:
— mobile phase A: mix 20 mL of a 100.0 g/L solution of triethylamine R2 previously adjusted to pH 3.0 using phosphoric acid R, and 50 mL of acetonitrile R; dilute to 1000 mL with water R;
— mobile phase B: mobile phase A, water R, acetonitrile R (2:6.5:91.5 V/V/V);
| Time
(min) |
Mobile phase A
(per cent V/V) |
Mobile phase B
(per cent V/V) |
| 0 – 15 | 45 | 55 |
| 15 – 30 | 45 → 5 | 55 → 95 |
| 30 – 40 | 5 | 95 |
Flow rate: 0.8 mL/min.
Detection: Spectrophotometer at 230 nm.
Injection: 10 μL.
Identification of impurities: Use the chromatogram obtained with reference solution (a) to identify the peaks due to impurities A and B; use the chromatogram supplied with glibenclamide for peak identification CRS and the chromatogram obtained with reference solution (c) to identify the peak due to impurity C.
Relative retention: With reference to glibenclamide (retention time = about 5 min): impurity A = about 0.5; impurity B = about 0.6; impurity C = about 0.7.
System suitability: Reference solution (a):
— resolution: minimum 2.0 between the peaks due to impurities A and B.
Limits:
— correction factor: for the calculation of content, multiply the peak area of impurity C by 1.8;
— impurity A: not more than the area of the corresponding peak in the chromatogram obtained with reference solution (a) (0.3 per cent);
— impurity C: not more than 1.5 times the area of the principal peak in the chromatogram obtained with reference solution (b) (0.15 per cent);
— unspecified impurities: for each impurity, not more than the area of the principal peak in the chromatogram obtained with reference solution (b) (0.10 per cent);
— total: 0.8 per cent;
— disregard limit: 0.5 times the area of the principal peak in the chromatogram obtained with reference solution (b) (0.05 per cent).
Loss on drying (2.2.32)
Maximum 1.0 per cent, determined on 1.000 g by drying in an oven at 105 °C.
Sulfated ash (2.4.14)
Maximum 0.1 per cent, determined on 1.0 g.
ASSAY
Dissolve 0.400 g with heating in 100 mL of ethanol (96 per cent) R. Titrate with 0.1 M sodium hydroxide, determining the end-point potentiometrically (2.2.20).
1 mL of 0.1 M sodium hydroxide is equivalent to 49.40 mg of C23H28ClN3O5S.
IMPURITIES
Specified impurities A, C.
Other detectable impurities (the following substances would, if present at a sufficient level, be detected by one or other of the tests in the monograph. They are limited by the general acceptance criterion for other/unspecified impurities and/or by the general monograph Substances for pharmaceutical use (2034). It is therefore not necessary to identify these impurities for demonstration of compliance. See also 5.10. Control of impurities in substances for pharmaceutical use) B, D, E.
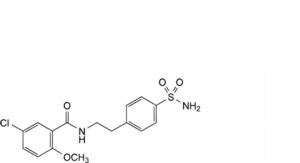
A. 5-chloro-2-methoxy-N-[2-(4-sulfamoylphenyl)ethyl]benzamide,
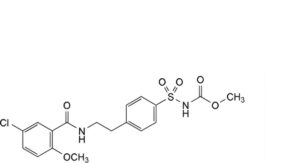
B. methyl [[4-[2-[(5-chloro-2-methoxybenzoyl)amino]ethyl]phenyl]sulfonyl]carbamate,
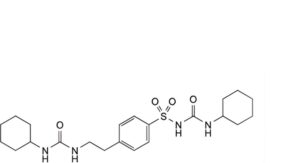
C. 1-cyclohexyl-3-[[4-[2-[(cyclohexylcarbamoyl)amino]ethyl]phenyl]sulfonyl]urea,
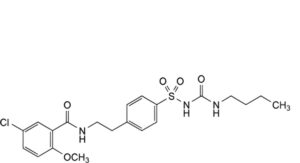
D. 1-butyl-3-[[4-[2-[(5-chloro-2-methoxybenzoyl)amino]ethyl]phenyl]sulfonyl]urea,
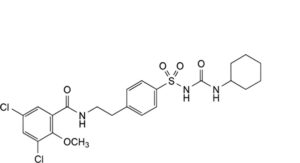
E. 1-cyclohexyl-3-[[4-[2-[(3,5-dichloro-2-methoxybenzoyl)amino]ethyl]phenyl]sulfonyl]urea.