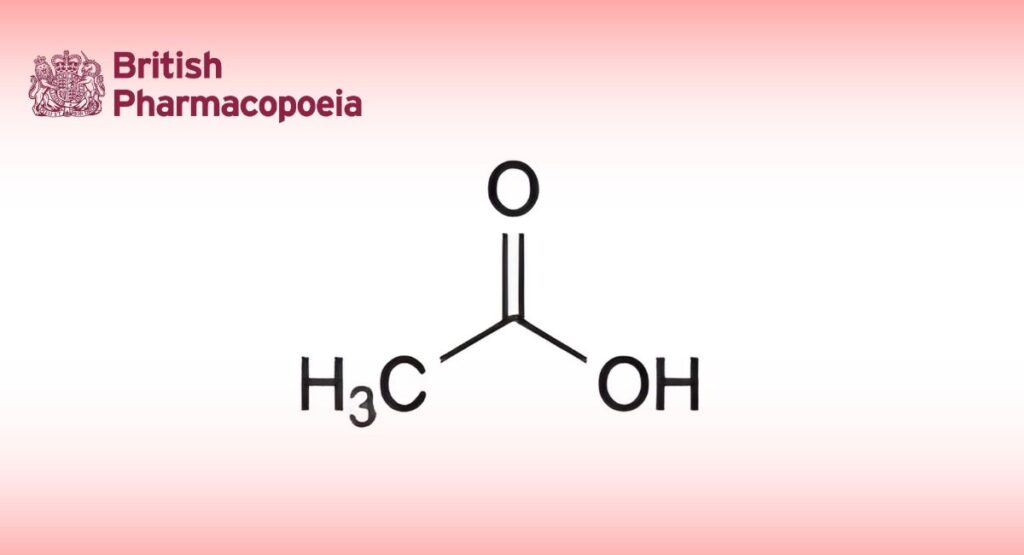
(Ph. Eur. monograph 0590)
C2H4O2 60.1 64-19-7
DEFINITION
Content
99.0 per cent m/m to 100.5 per cent m/m.
CHARACTERS
Appearance
Crystalline mass or clear, colourless, volatile liquid.
Solubility
Miscible with water, with ethanol (96 per cent) and with methylene chloride.
IDENTIFICATION
A. A 100 g/L solution is strongly acid (2.2.4).
B. To 0.03 mL add 3 mL of water R and neutralise with dilute sodium hydroxide solution R. The solution gives reaction (b) of acetates (2.3.1).
TESTS
Solution S
Dilute 20 mL to 100 mL with distilled water R.
Appearance
The substance to be examined is clear (2.2.1) and colourless (2.2.2, Method II).
Freezing point (2.2.18)
Minimum 14.8 °C.
Reducing substances
Dilute 2.0 mL to 10.0 mL with water R. Add 0.1 mL of 0.02 M potassium permanganate. Heat on a water-bath for 1 min, the colour remains pink.
Chlorides (2.4.4)
Maximum 25 mg/L.
Dilute 10 mL of solution S to 15 mL with water R.
Sulfates (2.4.13)
Maximum 50 mg/L, determined on solution S.
Iron (2.4.9)
Maximum 5 ppm.
Dissolve the residue obtained in the test for residue on evaporation by heating with 2 quantities, each of 15 mL, of water R and dilute to 50.0 mL with water R. Dilute 5.0 mL of the solution to 10.0 mL with water R.
Residue on evaporation
Maximum 0.01 per cent.
Evaporate 20 g to dryness on a water-bath and dry at 100-105 °C. The residue weighs a maximum of 2.0 mg.
ASSAY
Weigh accurately a conical flask with a ground-glass stopper containing 25 mL of water R. Add 1.0 mL of the substance to be examined and weigh again accurately. Add 0.5 mL of phenolphthalein solution R and titrate with 1 M sodium hydroxide.
1 mL of 1 M sodium hydroxide is equivalent to 60.1 mg of C2H4O2.
STORAGE
In an airtight container.