(Ph. Eur. monograph 2769)
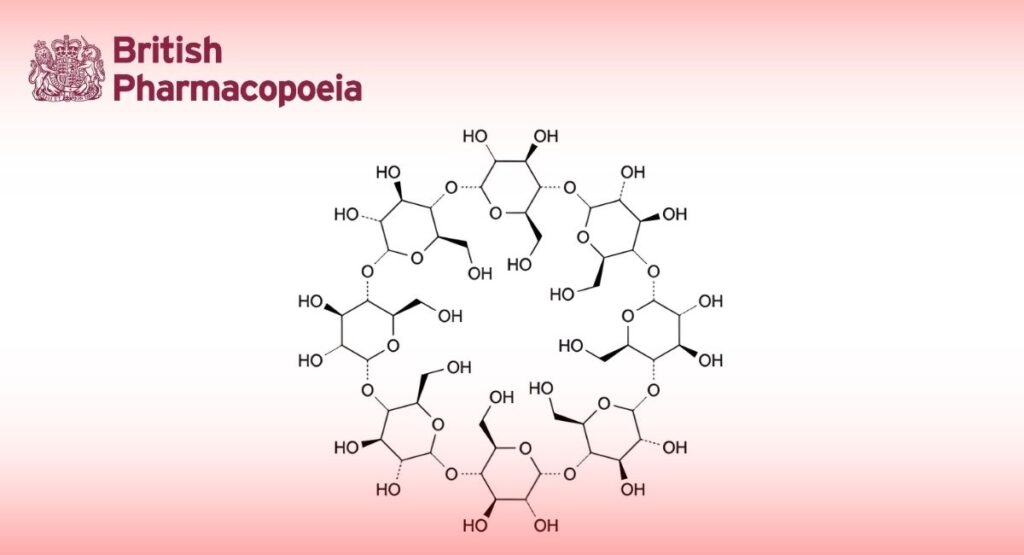
[C6H10O5]8 1297 17465-86-0DEFINITION
Cyclooctakis-(1→4)-(α-D-glucopyranosyl) (cyclomaltooctaose or γ-cyclodextrin).
Content
97.0 per cent to 102.0 per cent (dried substance).
CHARACTERS
Appearance
White or almost white, amorphous or crystalline, hygroscopic powder.
Solubility
Freely soluble in water, very slightly soluble in propylene glycol, practically insoluble in anhydrous ethanol and in methylene chloride. When dissolved in water, it forms a colloidal dispersion over time.
IDENTIFICATION
A. Specific optical rotation (2.2.7): + 174 to + 180 (dried substance), determined on solution S (see Tests) after filtration through a membrane filter (nominal pore size 0.45 μm).
B. Infrared absorption spectrophotometry (2.2.24).
Comparison: gammacyclodextrin CRS.
TESTS
Solution S
Dissolve 1.000 g in carbon dioxide-free water R and dilute to 100.0 mL with the same solvent.
pH (2.2.3)
5.0 to 8.0.
Mix 1 mL of a 223.6 g/L solution of potassium chloride R and 30 mL of solution S.
Reducing sugars
Maximum 0.2 per cent.
Test solution: To 1 mL of solution S add 1 mL of cupri-tartaric solution R4. Heat on a water-bath for 10 min, cool to room temperature. Add 10 mL of ammonium molybdate reagent R1 and allow to stand for 15 min.
Reference solution: Prepare a reference solution at the same time and in the same manner as the test solution, using 1 mL of a 0.02 g/L solution of glucose R.
Measure the absorbance (2.2.25) of the test solution and the reference solution at the absorption maximum at 740 nm using water R as the compensation liquid. The absorbance of the test solution is not greater than that of the reference solution.
Light-absorbing impurities
Examine solution S after filtration through a membrane filter (nominal pore size 0.45 μm). The absorbance (2.2.25) is not greater than 0.10 between 230 nm and 350 nm and not greater than 0.05 between 350 nm and 750 nm.
Related substances
Liquid chromatography (2.2.29).
Test solution (a): Dissolve 0.250 g of the substance to be examined in water R with heating, cool and dilute to 25.0 mL with the same solvent.
Test solution (b): Dissolve 25.0 mg of the substance to be examined in water R and dilute to 25.0 mL with the same solvent.
Reference solution (a): Dissolve 25.0 mg of alfadex CRS (impurity A), 25.0 mg of betadex CRS (impurity B) and 25.0 mg of gammacyclodextrin CRS (gammadex) in water R and dilute to 50.0 mL with the same solvent.
Reference solution (b): Dilute 5.0 mL of reference solution (a) to 50.0 mL with water R.
Reference solution (c): Dissolve 25.0 mg of gammacyclodextrin CRS in water R and dilute to 25.0 mL with the same solvent.
Column:
— size: l = 0.25 m, Ø = 4.6 mm;
— stationary phase: end-capped octadecylsilyl silica gel for chromatography R (5 μm);
— temperature: 30 °C.
Mobile phase: methanol R, water for chromatography R (5:95 V/V).
Flow rate: 1.5 mL/min.
Detection: Differential refractometer maintained at a constant temperature (e.g. 35 °C).
Equilibration: With the mobile phase for about 3 h.
Injection: 50 μL of test solution (a) and reference solutions (a) and (b).
Run time: 4 times the retention time of gammadex.
Identification of impurities: Use the chromatogram obtained with reference solution (a) to identify the peaks due to impurities A and B.
Relative retention: With reference to gammadex (retention time = about 7 min): impurity A = about 1.5; impurity B = about 3.2.
System suitability: Reference solution (a):
— resolution: minimum 2.5 between the peaks due to gammadex and impurity A;
— symmetry factor: 0.8 to 1.8 for the peak due to gammadex.
Calculation of percentage contents:
— for impurities A and B, use the concentration of the corresponding impurity in reference solution (b);
— for impurities other than A and B, use the concentration of gammadex in reference solution (b).
Limits:
— impurities A, B: for each impurity, maximum 0.5 per cent;
— sum of impurities other than A and B: maximum 0.5 per cent;
— reporting threshold: 0.15 per cent.
Loss on drying (2.2.32)
Maximum 11.0 per cent, determined on 1.000 g by drying in an oven at 120 °C for 2 h.
Sulfated ash (2.4.14)
Maximum 0.1 per cent, determined on 1.0 g.
ASSAY
Liquid chromatography (2.2.29) as described in the test for related substances with the following modifications.
Injection: Test solution (b) and reference solution (c).
System suitability: Reference solution (c):
— repeatability: maximum relative standard deviation of 2.0 per cent determined on 5 injections.
Calculate the percentage content of [C6H10O5]8 taking into account the assigned content of gammacyclodextrin CRS.
STORAGE
In an airtight container.
IMPURITIES
Specified impurities A, B.
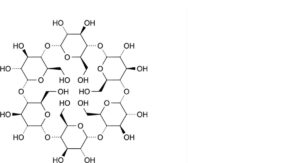
A. cyclohexakis-(1→4)-(α-D-glucopyranosyl) (alfadex or cyclomaltohexaose or α-cyclodextrin),
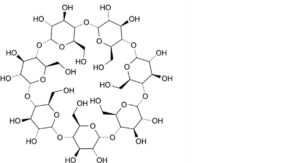
B. cycloheptakis-(1→4)-(α-D-glucopyranosyl) (betadex or cyclomaltoheptaose or β-cyclodextrin).