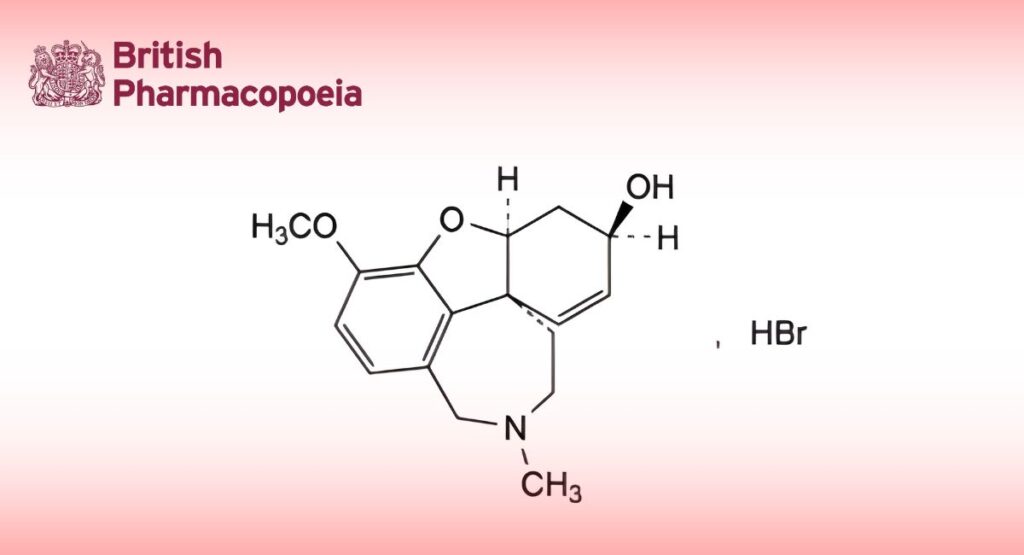
(Ph. Eur. monograph 2366)
C17H22BrNO3 368.3 1953-04-4
Action and use
Cholinesterase inhibitor; treatment of Alzheimer’s disease.
Preparations
Galantamine Oral Solution
Galantamine Prolonged-release Capsules
Galantamine Tablets
DEFINITION
(4aS,6R,8aS)-3-Methoxy-11-methyl-5,6,9,10,11,12-hexahydro-4aH-[1]benzofuro[3a,3,2-ef][2]benzazepin-6-ol hydrobromide.
It is isolated from natural sources or produced by a synthetic process.
Content
99.0 per cent to 101.0 per cent (dried substance).
CHARACTERS
Appearance
White or almost white, crystalline or amorphous powder.
Solubility
Sparingly soluble in water, very slightly soluble in anhydrous ethanol. It dissolves in dilute solutions of alkali hydroxides.
IDENTIFICATION
A. Infrared absorption spectrophotometry (2.2.24).
Comparison galantamine hydrobromide CRS.
B. Specific optical rotation or enantiomeric purity (see Tests).
C. It gives reaction (a) of bromides (2.3.1).
TESTS
Solution S
Dissolve 0.60 g in carbon dioxide-free water R and dilute to 30.0 mL with the same solvent.
pH (2.2.3)
4.0 to 5.5 for solution S.
Specific optical rotation (2.2.7)
For galantamine from natural sources: -100 to -90 (dried substance), determined on solution S.
Enantiomeric purity
For galantamine produced by a synthetic process. Capillary electrophoresis (2.2.47). Prepare the solutions immediately before use.
Buffer electrolyte 8.9 g/L solution of disodium hydrogen phosphate dihydrate R adjusted to pH 3.0 with phosphoric acid R.
Test solution: Dissolve 25.0 mg of the substance to be examined in 50.0 mL of water R and filter through a membrane filter (nominal pore size 0.22 μm).
Reference solution (a): Dissolve 5 mg of galantamine racemic mixture CRS in water R and dilute to 10 mL with the same solvent. Dilute 1 mL of the solution to 100 mL with water R and filter through a membrane filter (nominal pore size 0.22 μm).
Reference solution (b): Dilute 1.0 mL of the test solution to 100.0 mL with water R. Dilute 1.0 mL of this solution to 10.0 mL with water R and filter through a membrane filter (nominal pore size 0.22 μm).
Blank solution Filter water R through a membrane filter (nominal pore size 0.22 μm).
Capillary:
— material: uncoated fused silica;
— size: effective length = about 0.50 m, Ø = 75 μm.
Temperature 20 °C.
CZE buffer: Dissolve 0.196 g of α-cyclodextrin R in 10.0 mL of buffer electrolyte and filter through a membrane filter (nominal pore size 0.22 μm).
Detection: Spectrophotometer at 214 nm.
Preconditioning of the capillary At 137.9 kPa, rinse the capillary for 5 min with water R and for 5 min with CZE buffer.
Injection Under pressure (3.45 kPa) for 4 s.
Migration Apply a voltage of 15 kV.
Run time 35 min.
Relative migration times: With reference to galantamine (migration time = about 18 min): impurity F = about 1.05.
System suitability Reference solution (a):
— resolution: minimum 2.5 between the peaks due to galantamine and impurity F.
Limit:
— impurity F: not more than 1.5 times the area of the principal peak in the electropherogram obtained with reference solution (b) (0.15 per cent).
Related substances
Liquid chromatography (2.2.29).
A. Galantamine from natural sources
Solvent mixture: Mobile phase B, mobile phase A (10:90 V/V).
Test solution: Dissolve 12 mg of the substance to be examined in the solvent mixture and dilute to 10.0 mL with the solvent mixture.
Reference solution (a): Dilute 1.0 mL of the test solution to 100.0 mL with the solvent mixture. Dilute 1.0 mL of this solution to 10.0 mL with the solvent mixture.
Reference solution (b) Dissolve 5 mg of galantamine natural for system suitability CRS (containing impurities A and E) in the solvent mixture and dilute to 5 mL with the solvent mixture.
Column:
— size: l = 0.25 m, Ø = 4.6 mm;
— stationary phase: octylsilyl silica gel for chromatography R (5 μm);
— temperature: 30 °C.
Mobile phase:
— mobile phase A: dissolve 3.15 g of ammonium formate R in 900 mL of water for chromatography R, adjust to pH 3.8 with anhydrous formic acid R and dilute to 1000 mL with water for chromatography R;
— mobile phase B: acetonitrile R;
| Time (min) |
Mobile phase A (per cent V/V) |
Mobile phase B (per cent V/V) |
| 0 – 5 | 95 | 5 |
| 5 – 20 | 95 → 80 | 5 → 20 |
| 20 – 23 | 80 → 50 | 20 → 50 |
| 23 – 31 | 50 → 20 | 50 → 80 |
| 31 – 35 | 20 | 80 |
Flow rate 1.0 mL/min.
Detection Spectrophotometer at 287 nm.
Injection 10 μL.
Identification of impurities Use the chromatogram supplied with galantamine natural for system suitability CRS and the chromatogram obtained with reference solution (b) to identify the peaks due to impurities A and E.
Relative retention With reference to galantamine (retention time = about 12 min): impurity E = about 0.8;
impurity A = about 1.5.
System suitability Reference solution (b):
— resolution: minimum 5.0 between the peaks due to impurity E and galantamine.
Limits:
— impurity E: not more than 6 times the area of the principal peak in the chromatogram obtained with reference solution (a) (0.6 per cent);
— impurity A: not more than 1.5 times the area of the principal peak in the chromatogram obtained with reference solution (a) (0.15 per cent);
— unspecified impurities: for each impurity, not more than the area of the principal peak in the chromatogram obtained with reference solution (a) (0.10 per cent);
— total: not more than 8 times the area of the principal peak in the chromatogram obtained with reference
solution (a) (0.8 per cent);
— disregard limit: 0.5 times the area of the principal peak in the chromatogram obtained with reference solution (a) (0.05 per cent).
B. Galantamine produced by a synthetic process
Solvent mixture Dilute 50 mL of methanol R to 1000 mL with water R.
Test solution Dissolve 0.10 g of the substance to be examined in 50.0 mL of the solvent mixture and dilute to 100.0 mL with the solvent mixture.
Reference solution (a) Dilute 1.0 mL of the test solution to 100.0 mL with the solvent mixture. Dilute 5.0 mL of this solution to 10.0 mL with the solvent mixture.
Reference solution (b) Dissolve 2.5 mg of galantamine synthetic for system suitability CRS (containing impurities C and D) in the solvent mixture and dilute to 5 mL with the solvent mixture.
Column:
— size: l = 0.10 m, Ø = 4.6 mm;
— stationary phase: end-capped octadecylsilyl amorphous organosilica polymer for chromatography R (3.5 μm);
— temperature: 55 °C.
Mobile phase:
— mobile phase A: dissolve 0.79 g of disodium hydrogen phosphate dihydrate R and 2.46 g of sodium dihydrogen phosphate R in water for chromatography R and dilute to 1000 mL with the same solvent; to 950 mL of the solution add 50 mL of methanol R1;
— mobile phase B: acetonitrile for chromatography R;
| Time (min) |
Mobile phase A (per cent V/V) |
Mobile phase B (per cent V/V) |
| 0 – 6 | 100 | 0 |
| 6 – 20 | 100 → 95 | 0 → 5 |
| 20 – 35 | 95 → 85 | 5 → 15 |
| 35 – 50 | 85 → 80 | 15 → 20 |
Flow rate 1.5 mL/min.
Detection: Spectrophotometer at 230 nm.
Injection 20 μL.
Identification of impurities: Use the chromatogram supplied with galantamine synthetic for system suitability CRS and the chromatogram obtained with reference solution (b) to identify the peaks due to impurities C and D.
Relative retention: With reference to galantamine (retention time = about 16 min): impurity C = about 0.8;
impurity D = about 2.1.
System suitability Reference solution (b):
— resolution: minimum 4.5 between the peaks due to impurity C and galantamine.
Limits:
— impurities C, D: for each impurity, not more than 0.8 times the area of the principal peak in the chromatogram obtained with reference solution (a) (0.4 per cent);
— unspecified impurities: for each impurity, not more than 0.2 times the area of the principal peak in the
chromatogram obtained with reference solution (a) (0.10 per cent);
— total: not more than twice the area of the principal peak in the chromatogram obtained with reference solution (a) (1.0 per cent);
— disregard limit: 0.1 times the area of the principal peak in the chromatogram obtained with reference solution (a) (0.05 per cent).
Loss on drying (2.2.32)
Maximum 1.0 per cent, determined on 1.000 g in an oven at 105 °C for 4 h.
Sulfated ash (2.4.14)
Maximum 0.1 per cent, determined on 2.0 g.
ASSAY
Dissolve 0.275 g in 40 mL of water R. Add 40 mL of ethanol (96 per cent) R. Add 5 mL of 0.01 M hydrochloric acid. Titrate with 0.1 M sodium hydroxide, determining the end-point potentiometrically (2.2.20). Read the volume added between the 2 points of inflexion.
1 mL of 0.1 M sodium hydroxide is equivalent to 36.83 mg of C17H22BrNO3.
LABELLING
The label states the origin of the substance:
— isolated from natural sources;
— produced by a synthetic process.
IMPURITIES
Test A for related substances A, B, E.
Specified impurities A, E. Other detectable impurities (the following substances would, if present at a sufficient level, be detected by one or other of the tests in the monograph. They are limited by the general acceptance criterion for other/unspecified impurities and/or by the general monograph Substances for pharmaceutical use (2034). It is therefore not necessary to identify these impurities for demonstration of compliance. See also 5.10. Control of impurities in substances for pharmaceutical use) B.
Test B for related substances A, B, C, D, E, F.
Specified impurities C, D, F.
Other detectable impurities (the following substances would, if present at a sufficient level, be detected by one or other of the tests in the monograph. They are limited by the general acceptance criterion for other/unspecified impurities and/or by the general monograph Substances for pharmaceutical use (2034). It is therefore not necessary to identify these impurities for demonstration of compliance. See also 5.10. Control of impurities in substances for pharmaceutical use) A, B, E.
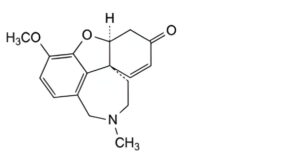
A. (4aS,8aS)-3-methoxy-11-methyl-4a,5,9,10,11,12-hexahydro-6H-[1]benzofuro[3a,3,2-ef][2]benzazepin-6-one (narwedine),
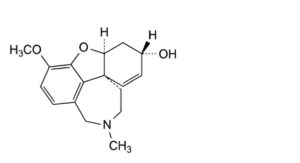
B. (4aS,6S,8aS)-3-methoxy-11-methyl-5,6,9,10,11,12-hexahydro-4aH-[1]benzofuro[3a,3,2-ef][2]benzazepin-6-ol (epi-galantamine),
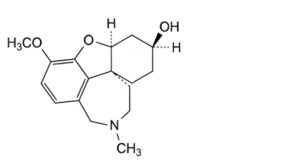
C. (4aS,6S,8aR)-3-methoxy-11-methyl-5,6,7,8,9,10,11,12-octahydro-4aH-[1]benzofuro[3a,3,2-ef][2]benzazepin-6-ol (dihydrogalantamine),
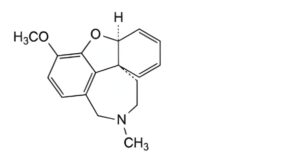
D. (4aS,8aS)-3-methoxy-11-methyl-9,10,11,12-tetrahydro-4aH-[1]benzofuro[3a,3,2-ef][2]benzazepine
(anhydrogalantamine),
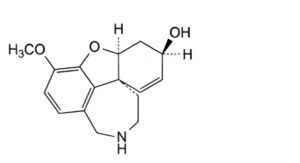
E. (4aS,6R,8aS)-3-methoxy-5,6,9,10,11,12-hexahydro-4aH-[1]benzofuro[3a,3,2-ef][2]benzazepin-6-ol (N-
demethylgalantamine),
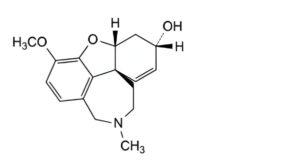
F. (4aR,6S,8aR)-3-methoxy-11-methyl-5,6,9,10,11,12-hexahydro-4aH-[1]benzofuro[3a,3,2-ef][2]benzazepin-6-ol (ent-galantamine).