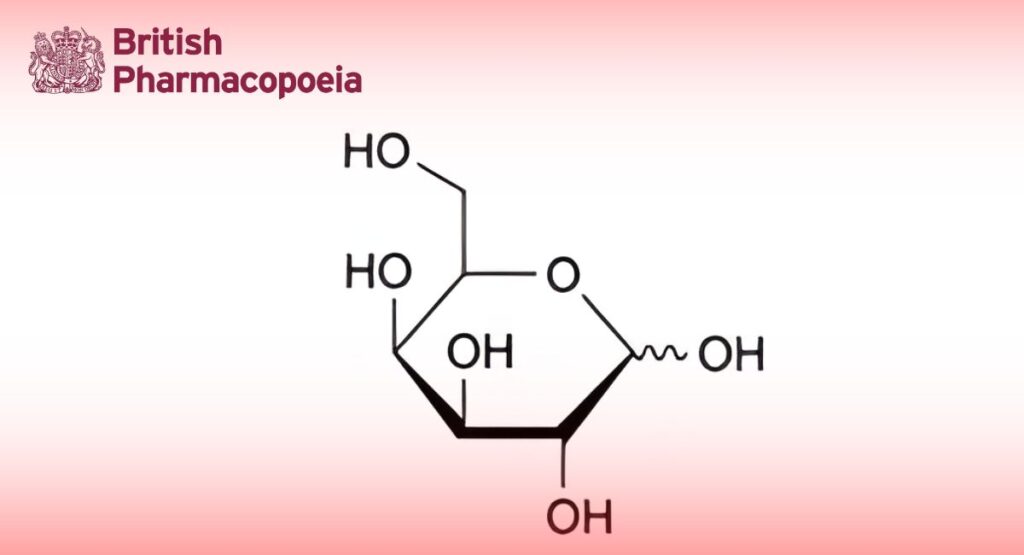
(Ph. Eur. monograph 1215)
C6H12O6 180.2 59-23-4
DEFINITION
D-Galactopyranose.
Content
97.0 per cent to 102.0 per cent (anhydrous substance).
CHARACTERS
Appearance
White or almost white, crystalline or finely granulated powder.
Solubility
Freely soluble or soluble in water, very slightly soluble in ethanol (96 per cent).
IDENTIFICATION
First identification: A.
Second identification: B, C.
A. Infrared absorption spectrophotometry (2.2.24).
Comparison: galactose CRS.
B. Thin-layer chromatography (2.2.27).
Test solution: Dissolve 10 mg of the substance to be examined in a mixture of 2 volumes of water R and 3 volumes of methanol R and dilute to 20 mL with the same mixture of solvents.
Reference solution (a): Dissolve 10 mg of galactose CRS in a mixture of 2 volumes of water R and 3 volumes of methanol R and dilute to 20 mL with the same mixture of solvents.
Reference solution (b): Dissolve 10 mg each of galactose R, glucose R and lactose monohydrate R (impurity A) in a mixture of 2 volumes of water R and 3 volumes of methanol R and dilute to 20 mL with the same mixture of solvents.
Plate: TLC silica gel plate R.
Mobile phase: water R, propanol R (15:85 V/V).
Application: 2 μL; thoroughly dry the points of application.
Development: In an unsaturated tank over 3/4 of the plate.
Drying: In a current of warm air.
Detection: Spray with a solution of 0.5 g of thymol R in a mixture of 5 mL of sulfuric acid R and 95 mL of ethanol (96 per cent) R. Heat in an oven at 130 °C for 10 min.
System suitability: Reference solution (b):
— the chromatogram shows 3 clearly separated spots.
Results: The principal spot in the chromatogram obtained with the test solution is similar in position, colour and size to the principal spot in the chromatogram obtained with reference solution (a).
C. Dissolve 0.1 g in 10 mL of water R. Add 3 mL of cupri-tartaric solution R and heat. An orange or red precipitate is formed.
TESTS
Solution S
Dissolve, with heating in a water-bath at 50 °C, 10.0 g in carbon dioxide-free water R prepared from distilled water R and dilute to 50 mL with the same solvent.
Appearance of solution
Solution S is clear (2.2.1) and not more intensely coloured than reference solution B8 (2.2.2, Method II).
Acidity or alkalinity
To 30 mL of solution S add 0.3 mL of phenolphthalein solution R. The solution is colourless. Not more than 1.5 mL of 0.01 M sodium hydroxide is required to change the colour of the indicator to pink.
Proteins
Maximum 0.1 mg/mL.
Dissolve 1.000 g of the substance to be examined in water R and dilute to 10.0 mL with the same solvent. Measure the absorbance (2.2.25) of the solution at 260 nm and at 280 nm and calculate the protein content, in milligrams per millilitre, using the following expression:
(A280 × 1.45) − (A260 × 0.74)
A280 = absorbance at 280 nm;
A260 = absorbance at 260 nm.
Related substances
Liquid chromatography (2.2.29).
Test solution (a): Dissolve 0.250 g of the substance to be examined in water R and dilute to 10.0 mL with the same solvent.
Test solution (b): Dilute 1.0 mL of test solution (a) to 50.0 mL with water R.
Reference solution (a): Dilute 1.0 mL of test solution (a) to 100.0 mL with water R.
Reference solution (b): Dissolve 3 mg each of 1,6-galactosylgalactose R (impurity B), galacturonic acid R (impurity C) and lactose monohydrate R (impurity A) in water R and dilute to 10 mL with the same solvent. To 100 μL of the solution add 900 μL of test solution (a).
Reference solution (c): Dissolve 25.0 mg of galactose CRS in water R and dilute to 50.0 mL with the same solvent.
Column 2 columns to be connected in series:
— size: l = 0.30 m, Ø = 6.5 mm;
— stationary phase: strong cation-exchange resin (calcium form) R (10 μm);
— temperature: 75 °C.
Mobile phase: Dissolve 50 mg of sodium calcium edetate R in 900 mL of water for chromatography R, add 1.0 mL of sulfuric acid R and dilute to 1000 mL with water for chromatography R.
Flow rate: 0.4 mL/min.
Detection: Differential refractometer maintained at a constant temperature (about 40 °C).
Injection: 10 μL of test solution (a) and reference solutions (a) and (b).
Run time: Twice the retention time of galactose.
Identification of impurities: Use the chromatogram obtained with reference solution (b) to identify the peaks due to impurities A + B and C.
Relative retention: With reference to galactose (retention time = about 24 min): impurities A and B = about 0.8; impurity C = about 0.9.
System suitability: Reference solution (b):
— resolution: minimum 2.0 between the peaks due to impurity C and galactose.
Calculation of percentage contents:
— for each impurity, use the concentration of galactose in reference solution (a).
Limits:
— sum of impurities A and B: maximum 1.0 per cent;
— unspecified impurities: for each impurity, maximum 0.3 per cent;
— total: maximum 2.0 per cent;
— reporting threshold: 0.2 per cent.
Water (2.5.12)
Maximum 1.0 per cent, determined on 1.00 g.
Sulfated ash
Maximum 0.1 per cent.
To 5 mL of solution S add 2 mL of sulfuric acid R, evaporate to dryness on a water-bath and ignite to constant mass. The residue weighs a maximum of 1 mg.
Microbial contamination
TAMC: acceptance criterion 10 CFU/g (2.6.12).
ASSAY
Liquid chromatography (2.2.29) as described in the test for related substances with the following modification.
Injection: 10 μL of test solution (b) and reference solution (c).
Calculate the percentage content of C6H12O6 taking into account the assigned content of galactose CRS.
IMPURITIES
Specified impurities A, B.
Other detectable impurities (the following substances would, if present at a sufficient level, be detected by one or other of the tests in the monograph. They are limited by the general acceptance criterion for other/unspecified impurities. It is therefore not necessary to identify these impurities for demonstration of compliance. See also 5.10. Control of impurities in substances for pharmaceutical use) C, D, E.
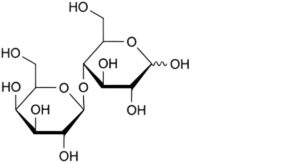
A. 4-O-β-D-galactopyranosyl-D-glucopyranose (lactose),
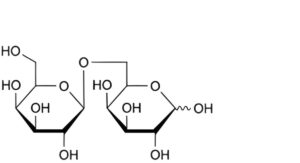
B. 6-O-β-D-galactopyranosyl-D-galactopyranose (1,6-galactosylgalactose),
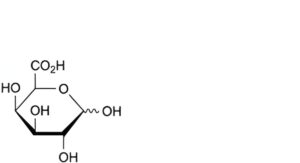
C. D-galactopyranuronic acid (galacturonic acid),
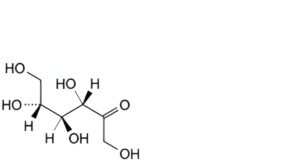
D. D-tagatose,
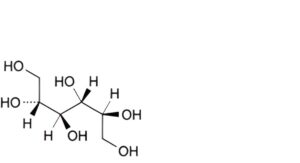
E. galactitol (dulcitol).