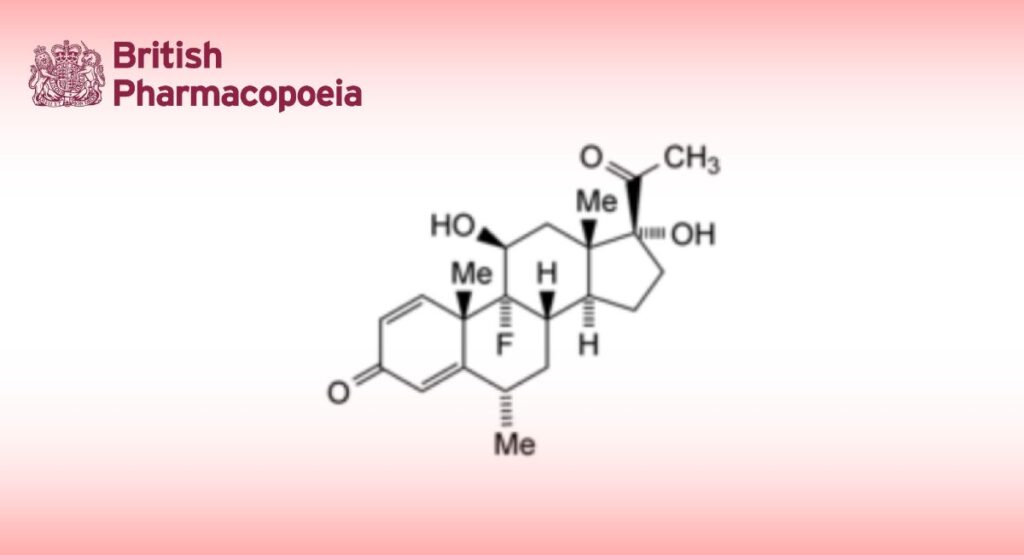
C22H29FO4 376.5 426-13-1
Action and use
Glucocorticoid.
Preparation
Fluorometholone Eye Drops
DEFINITION
Fluorometholone is 9α-fluoro-11β,17α-dihydroxy-6α-methylpregna-1,4-diene-3,20-dione. It contains not less than 97.0% and not more than 103.0% of C22H29FO4, calculated with reference to the dried substance.
CHARACTERISTICS
A white to yellowish white, crystalline powder. It melts at about 280°, with decomposition.
Practically insoluble in water; slightly soluble in absolute ethanol and in ether.
IDENTIFICATION
A. The infrared absorption spectrum, Appendix II A, is concordant with the reference spectrum of fluorometholone (RS152).
B. In the Assay, the principal peak in the chromatogram obtained with solution (1) has the same retention time as the principal peak in the chromatogram obtained with solution (2).
TESTS
Specific optical rotation
In a 1% w/v solution in pyridine, +52 to +60, Appendix V F, calculated with reference to the dried substance.
Related substances
Carry out the method for liquid chromatography, Appendix III D, using the following solutions in methanol.
(1) 0.010% w/v of the substance being examined.
(2) 0.00005% w/v of the substance being examined.
(3) 0.00005% w/v each of deltamedrane BPCRS and fluorometholone BPCRS.
CHROMATOGRAPHIC CONDITIONS
(a) Use a stainless steel column (30 cm × 3.9 mm) packed with octadecylsilyl silica gel for chromatography (10 μm) (μBondapak C18 is suitable).
(b) Use isocratic elution and the mobile phase described below.
(c) Use a flow rate of 2 mL per minute.
(d) Use an ambient column temperature.
(e) Use a detection wavelength of 254 nm.
(f) Inject 20 μL of each solution.
MOBILE PHASE
40 volumes of water and 60 volumes of methanol.
SYSTEM SUITABILITY
The test is not valid unless, in the chromatogram obtained with solution (3), the resolution factor between the peaks due to deltamedrane and fluorometholone is at least 1.5.
LIMITS
In the chromatogram obtained with solution (1):
the area of any secondary peak is not greater than the area of the principal peak in the chromatogram obtained with solution (2) (0.5%); the sum of the areas of any secondary peaks is not greater than twice the area of the principal peak in the chromatogram obtained with solution (2) (1%).
Loss on drying
When dried at 60° at a pressure not exceeding 0.7 kPa for 3 hours, loses not more than 0.5% of its weight. Use 1 g.
Sulfated ash
Not more than 0.1%, Appendix IX A.
ASSAY
Carry out the method for liquid chromatography, Appendix III D, using the following solutions in methanol.
(1) 0.005% w/v of the substance being examined.
(2) 0.005% w/v of fluorometholone BPCRS.
(3) 0.00005% w/v each of deltamedrane BPCRS and fluorometholone BPCRS.
CHROMATOGRAPHIC CONDITIONS
The chromatographic conditions described under Related substances may be used.
SYSTEM SUITABILITY
The test is not valid unless, in the chromatogram obtained with solution (3), the resolution factor between the peaks due to deltamedrane and fluorometholone is at least 1.5.
DETERMINATION OF CONTENT
Calculate the content of C22H29FO4 from the chromatograms obtained and using the declared content of C22H29FO4 in fluorometholone BPCRS.
IMPURITIES
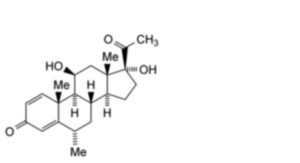
A. 11β,17α-dihydroxy-6α-methylpregna-1,4-diene-3,20-dione (deltamedrane),
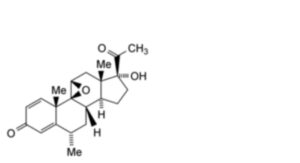
B. 9β,11β-epoxy-17α-hydroxy-6α-methylpregna-1,4-diene-3,20-dione (epoxymedradiene).