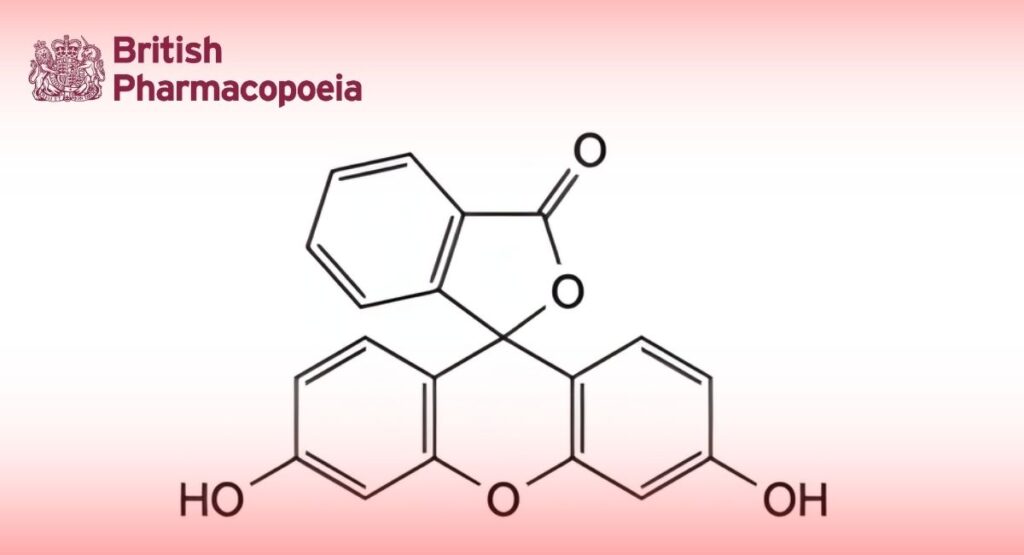
(Ph. Eur. monograph 2348)
C20H12O5 332.3 2321-07-5
Action and use
Detection of corneal lesions, retinal angiography and pancreatic function testing.
DEFINITION
3′,6′-Dihydroxy-3H-spiro[isobenzofuran-1,9′-xanthen]-3-one.
Content
97.0 per cent to 102.0 per cent (dried substance).
CHARACTERS
Appearance
Orange-red, fine powder.
Solubility
Practically insoluble in water, soluble in hot ethanol (96 per cent). It dissolves in dilute solutions of alkali hydroxides.
IDENTIFICATION
First identification: A, D.
Second identification: B, C, D.
A. Infrared absorption spectrophotometry (2.2.24).
Comparison: fluorescein CRS.
Dissolve the substance to be examined and the reference substance separately in the minimum volume of ethanol (96 per cent) R, evaporate to dryness and record the spectra using the residues.
B. Dilute 0.1 mL of solution S (see Tests) to 10 mL with water R. The solution shows a yellowish-green fluorescence. The fluorescence disappears on addition of 0.1 mL of dilute hydrochloric acid R and reappears on addition of 0.2 mL of dilute sodium hydroxide solution R.
C. The absorption by a piece of filter paper of 0.05 mL of the solution prepared for identification B (before the addition of dilute hydrochloric acid R) colours the paper yellow. On exposing the moist paper to bromine vapour for 1 min and then to ammonia vapour, the colour becomes deep pink.
D. Suspend 0.5 g in 50 mL of water R and shake for 10 min. The substance does not completely dissolve.
TESTS
Solution S
Suspend 1.0 g in 35.0 mL of water R and add dropwise with shaking 4.5 mL of 1 M sodium hydroxide. Adjust to pH 8.5-9.0 with 1 M sodium hydroxide and dilute to 50.0 mL with water R to obtain a clear solution.
Appearance of solution
Solution S is clear (2.2.1) and orange-red with yellowish-green fluorescence.
Related substances
Liquid chromatography (2.2.29).
Solvent mixture acetonitrile for chromatography R, mobile phase A (30:70 V/V).
Test solution (a): Disperse 50.0 mg of the substance to be examined in 15.0 mL of ethanol (96 per cent) R.
Sonicate and dilute to 50.0 mL with the solvent mixture.
Test solution (b): Dilute 5.0 mL of test solution (a) to 250.0 mL with the solvent mixture.
Reference solution (a): Disperse 50.0 mg of fluorescein CRS in 15.0 mL of ethanol (96 per cent) R. Sonicate and dilute to 50.0 mL with the solvent mixture. Dilute 5.0 mL of this solution to 250.0 mL with the solvent mixture.
Reference solution (b): Dissolve 10.0 mg of phthalic acid CRS (impurity B) and 10.0 mg of resorcinol CRS (impurity A) in the solvent mixture and dilute to 100.0 mL with the solvent mixture. Dilute 1.0 mL of this solution to 100.0 mL with the solvent mixture.
Reference solution (c): Dilute 5.0 mL of test solution (b) to 20.0 mL with the solvent mixture.
Reference solution (d): Dilute 10.0 mL of reference solution (c) to 100.0 mL with the solvent mixture.
Reference solution (e): Dissolve the contents of a vial of fluorescein impurity C CRS in 1 mL of the solvent mixture.
Column:
— size: l = 0.25 m, Ø = 4.6 mm;
— stationary phase: octylsilyl silica gel for chromatography R3 (5 μm);
— temperature: 35 °C.
Mobile phase:
— mobile phase A: dissolve 0.610 g of potassium dihydrogen phosphate R in water for chromatography R, adjust to pH 2.0 with phosphoric acid R and dilute to 1000.0 mL with water for chromatography R;
— mobile phase B: acetonitrile for chromatography R;
| Time
(min) |
Mobile phase A
(per cent V/V) |
Mobile phase B
(per cent V/V) |
| 0 – 20 | 85 → 20 | 15 → 80 |
| 20 – 29 | 20 | 80 |
Flow rate: 1.0 mL/min.
Detection: Spectrophotometer at 220 nm.
Injection: 20 μL of test solution (a) and reference solutions (b), (c), (d) and (e).
Identification of impurity C: Use the chromatogram obtained with reference solution (e) to identify the peak due to
impurity C.
Relative retention: With reference to fluorescein (retention time = about 15 min): impurity A = about 0.42; impurity B = about 0.48; impurity C = about 0.86.
System suitability: Reference solution (b):
— resolution: minimum 2.0 between the peaks due to impurities A and B.
Limits:
— correction factor: for the calculation of content, multiply the peak area of impurity C by 1.9;
— impurity C: not more than 1.2 times the area of the principal peak in the chromatogram obtained with reference solution (c) (0.6 per cent);
— impurities A, B: for each impurity, not more than the area of the corresponding peak in the chromatogram obtained with reference solution (b) (0.1 per cent);
— unspecified impurities: for each impurity, not more than 0.2 times the area of the principal peak in the chromatogram obtained with reference solution (c) (0.10 per cent);
— sum of impurities other than A, B and C: not more than 0.4 times the area of the principal peak in the chromatogram obtained with reference solution (c) (0.2 per cent);
— disregard limit: the area of the principal peak in the chromatogram obtained with reference solution (d) (0.05 per cent).
Chlorides (2.4.4)
Maximum 0.25 per cent.
To 10.0 mL of solution S add 90.0 mL of water R and 3.0 mL of dilute nitric acid R, wait for at least 30 min and filter. Dilute 10.0 mL of the filtrate to 15.0 mL with water R.
Loss on drying (2.2.32)
Maximum 1.0 per cent, determined on 1.000 g by drying in an oven at 105 °C.
ASSAY
Liquid chromatography (2.2.29) as described in the test for related substances with the following modification.
Injection: Test solution (b) and reference solution (a).
Calculate the percentage content of C20H12O5 taking into account the assigned content of fluorescein CRS.
STORAGE
Protected from light.
IMPURITIES
Specified impurities A, B, C.
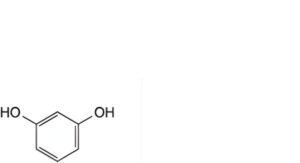
A. benzene-1,3-diol (resorcinol),
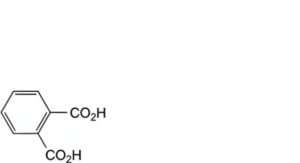
B. benzene-1,2-dicarboxylic acid (phthalic acid),
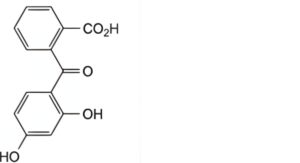
C. 2-(2,4-dihydroxybenzoyl)benzoic acid.