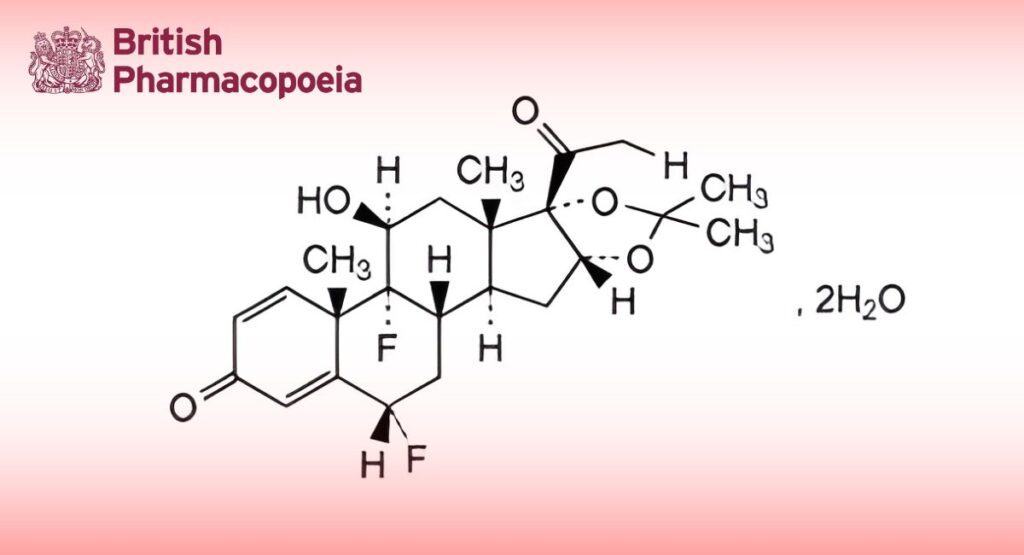
C24H30F2O6,2H2O 488.5 (anhydrous) 67-73-2
Action and use
Glucocorticoid.
Preparations
Fluocinolone Cream
Fluocinolone Ointment
DEFINITION
Fluocinolone Acetonide Dihydrate is 6α,9α-difluoro-11β,21-dihydroxy-16α-,17α-isopropylidenedioxypregna-1,4-diene-3,20- dione dihydrate. It contains not less than 96.0% and not more than 104.0% of C24H30F2O6, calculated with reference to the anhydrous substance.
CHARACTERISTICS
A white or almost white, crystalline powder.
Practically insoluble in water; freely soluble in acetone; soluble in absolute ethanol; sparingly soluble in dichloromethane and in methanol; practically insoluble in hexane.
IDENTIFICATION
A. The infrared absorption spectrum, Appendix II A, is concordant with the reference spectrum of fluocinolone acetonide dihydrate (RS 147).
B. Complies with the test for identification of steroids, Appendix III A, using impregnating solvent I and mobile phase H. Apply 5 μL of each of the three solutions.
C. Complies with the test for identification of steroids, Appendix III A, using the conditions specified in test B but using solutions prepared in the following manner. For solution (1) dissolve 10 mg in 1.5 mL of glacial acetic acid in a separating funnel, add 0.5 mL of a 2% w/v solution of chromium(VI) oxide and allow to stand for 30 minutes. Add 5 mL of water and 2 mL of dichloromethane and shake vigorously for 2 minutes. Allow to separate and use the lower layer. Prepare solution (2) in the same manner but using 10 mg of fluocinolone acetonide BPCRS.
TESTS
Light absorption
Dissolve 15 mg in sufficient absolute ethanol to produce 100 mL. Dilute 10 mL of the solution to 100 mL with absolute ethanol. The A(1%, 1 cm) of the resulting solution at the maximum at 239 nm is 345 to 375, calculated with reference to the anhydrous substance, Appendix II B.
Specific optical rotation
In a 1% w/v solution in 1,4-dioxan, +92 to +96, calculated with reference to the anhydrous substance, Appendix V F.
Related substances
Carry out the method for liquid chromatography, Appendix III D, using the following solutions.
(1) 0.25% w/v of the substance being examined in acetonitrile.
(2) 0.025% w/v each of fluocinolone acetonide BPCRS and triamcinolone acetonide BPCRS in 45% w/v of acetonitrile.
(3) Dilute 1 volume of solution (1) to 100 volumes with acetonitrile.
(4) Dilute 1 volume of solution (3) to 20 volumes with acetonitrile.
CHROMATOGRAPHIC CONDITIONS
(a) Use a stainless steel column (25 cm × 4.6 mm) packed with base-deactivated end-capped octadecylsilyl silica gel for chromatography (5 μm) (Hypersil BDS is suitable).
(b) Use isocratic elution and the mobile phase described below.
(c) Use a flow rate of 1 mL per minute.
(d) Use an ambient column temperature.
(e) Use a detection wavelength of 238 nm.
(f) Inject 20 μL of each solution.
(g) Allow the chromatography to proceed for 4 times the retention time of the principal peak.
MOBILE PHASE
45 volumes of acetonitrile and 55 volumes of water.
SYSTEM SUITABILITY
The test is not valid unless:
in the chromatogram obtained with solution (2), the resolution factor between the peaks due to triamcinolone acetonide and fluocinolone acetonide is at least 3.0; in the chromatogram obtained with solution (4), the signal-to-noise ratio of the principal peak is at least 10.
LIMITS
In the chromatogram obtained with solution (1):
the area of any secondary peak is not greater than the area of the principal peak in the chromatogram obtained with solution (3) (1%);
the area of not more than one secondary peak is greater than 0.5 times the area of the principal peak in the chromatogram obtained with solution (3) (0.5%);
the sum of the areas of any secondary peaks is not greater than 2.5 times the area of the principal peak in the chromatogram obtained with solution (3) (2.5%).
Disregard any peak with an area less than the area of the principal peak in the chromatogram obtained with solution (4) (0.05%).
Water
7.0 to 8.5% w/w, Appendix IX C. Use 0.5 g.
ASSAY
Carry out the tetrazolium assay of steroids, Appendix VIII J, and calculate the content of C24H30F2O6 from the absorbance obtained by repeating the operation using fluocinolone acetonide BPCRS in place of the substance being examined.
STORAGE
Fluocinolone Acetonide Dihydrate should be protected from light.