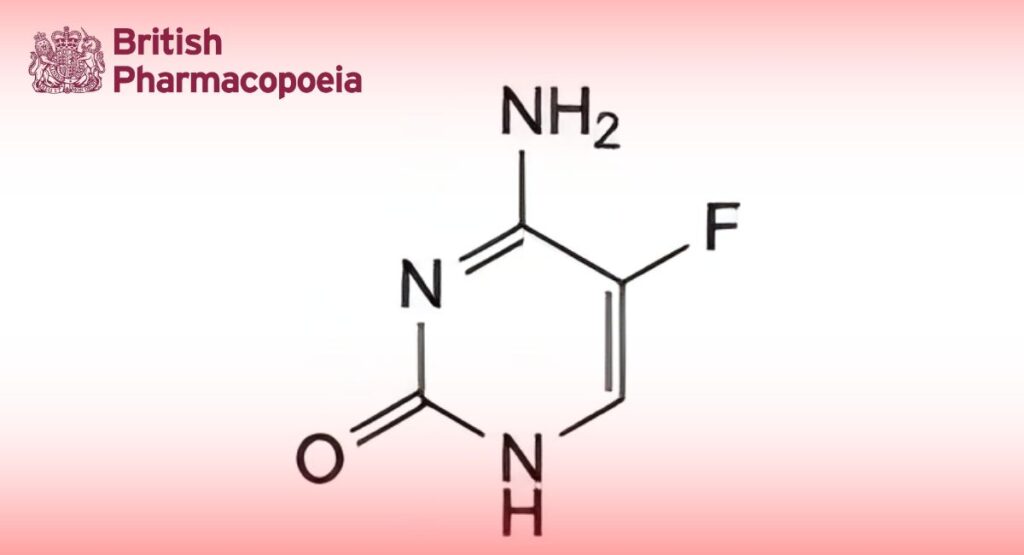
(Ph. Eur. monograph 0766)
C4H4FN3O 129.1 2022-85-7
Action and use
Antifungal.
DEFINITION
4-Amino-5-fluoropyrimidin-2(1H)-one.
Content
99.0 per cent to 101.0 per cent (dried substance).
CHARACTERS
Appearance
White or almost white, crystalline powder.
Solubility
Sparingly soluble in water, slightly soluble in ethanol (96 per cent).
IDENTIFICATION
First identification: A.
Second identification: B, C, D.
A. Infrared absorption spectrophotometry (2.2.24).
Comparison: flucytosine CRS.
B. Thin-layer chromatography (2.2.27).
Solvent mixture: water R, methanol R (10:15 V/V).
Test solution: Dissolve 10 mg of the substance to be examined in the solvent mixture and dilute to 10 mL with the solvent mixture.
Reference solution: Dissolve 10 mg of flucytosine CRS in the solvent mixture and dilute to 10 mL with the solvent mixture.
Plate: TLC silica gel F254 plate R.
Mobile phase: anhydrous formic acid R, water R, methanol R, ethyl acetate R (1:15:25:60 V/V/V/V).
Application: 10 μL.
Development: Over 2/3 of the plate in an unsaturated tank with the mobile phase. Then allow the solvents to evaporate.
Detection: At the bottom of a chromatography tank place an evaporating dish containing a mixture of 1 volume of hydrochloric acid R1, 1 volume of water R and 2 volumes of a 15 g/L solution of potassium permanganate R. Close the tank and allow to stand for 15 min. Place the dried plate in the tank and close the tank. Leave the plate in contact with the
chlorine vapour for 5 min. Withdraw the plate and place it in a current of cold air until the excess of chlorine is removed and an area of the coating below the points of application does not give a blue colour with a drop of potassium iodide and starch solution R. Spray with potassium iodide and starch solution R. Examine in daylight.
Results: The principal spot in the chromatogram obtained with the test solution is similar in position and size to the principal spot in the chromatogram obtained with the reference solution.
C. Mix about 5 mg with 45 mg of heavy magnesium oxide R and ignite in a crucible until an almost white residue is obtained (usually less than 5 min). Allow to cool, add 1 mL of water R, 0.05 mL of phenolphthalein solution R1 and about 1 mL of dilute hydrochloric acid R to render the solution colourless. Filter and add to the filtrate a freshly prepared mixture
of 0.1 mL of alizarin S solution R and 0.1 mL of zirconyl nitrate solution R. Mix, allow to stand for 5 min and compare the colour of the solution with that of a blank prepared in the same manner. The colour of the solution changes from red to yellow.
D. To 5 mL of solution S (see Tests) add 0.15 mL of bromine water R and shake. The colour of the solution is discharged.
TESTS
Solution S
Dissolve 0.5 g in carbon dioxide-free water R and dilute to 50 mL with the same solvent.
Appearance of solution
Solution S is clear (2.2.1) and not more intensely coloured than reference solution BY7 or Y7 (2.2.2, Method II).
Related substances
Liquid chromatography (2.2.29).
Solvent mixture: Dissolve 13.6 g of potassium dihydrogen phosphate R in 950 mL of water R. Add 50 mL of methanol R. Mix thoroughly.
Test solution: Dissolve 15.0 mg of the substance to be examined in the solvent mixture and dilute to 50.0 mL with the solvent mixture. Mix well. Sonicate for 5 min. Mix thoroughly. Sonicate the solution for 5 min. Mix thoroughly.
Reference solution (a): Dilute 1.0 mL of the test solution to 100.0 mL with the solvent mixture. Dilute 1.0 mL of this solution to 10.0 mL with the solvent mixture.
Reference solution (b): Dissolve 15.0 mg of fluorouracil CRS (impurity A) in the solvent mixture and dilute to 50.0 mL with the solvent mixture. Mix well. Sonicate for 5 min. Mix thoroughly. Sonicate the solution for 5 min. Mix thoroughly. Dilute 1.0 mL of this solution to 10.0 mL with the solvent mixture. Dilute 2.0 mL of this solution to 100.0 mL with the solvent
mixture.
Reference solution (c): Dissolve the contents of a vial of flucytosine for system suitability CRS (containing impurity B) in 0.5 mL of the solvent mixture and add 0.5 mL of reference solution (b).
Column:
— size: l = 0.25 m, Ø = 4.0 mm;
— stationary phase: end-capped octadecylsilyl silica gel for chromatography R (5 μm).
Mobile phase: Dissolve 13.6 g of potassium dihydrogen phosphate R in 950 mL of water R. Filter through a membrane filter (nominal pore size 0.45 μm). Adjust to pH 2.0 by adding phosphoric acid R and add 50 mL of methanol R. Mix thoroughly.
Flow rate: 1.1 mL/min.
Detection: Spectrophotometer at 260 nm.
Injection: 20 μL of the test solution and reference solutions (a) and (c).
Run time: 15 times the retention time of flucytosine.
Identification of impurities: Use the chromatogram obtained with reference solution (c) to identify the peaks due to impurities A and B.
Relative retention: With reference to flucytosine (retention time = about 2 min): impurity A = about 1.7; impurity B = about 13.3.
System suitability:
— resolution: minimum 5.0 between the peaks due to flucytosine and impurity A in the chromatogram obtained with reference solution (c);
— signal-to-noise ratio: minimum 50 for the peak due to impurity B in the chromatogram obtained with reference solution (c);
— symmetry factor: maximum 2.0 for the peak due to flucytosine in the chromatogram obtained with reference solution (a).
Limits:
— correction factor: for the calculation of content, multiply the peak area of impurity B by 0.6;
— impurity A: not more than 1.5 times the area of the corresponding peak in the chromatogram obtained with reference solution (c) (0.15 per cent);
— impurity B: not more than 1.5 times the area of the principal peak in the chromatogram obtained with reference solution (a) (0.15 per cent);
— unspecified impurities: for each impurity, not more than 0.5 times the area of the principal peak in the chromatogram obtained with reference solution (a) (0.05 per cent);
— total: not more than 3 times the area of the principal peak in the chromatogram obtained with reference solution (a) (0.3 per cent);
— disregard limit: 0.3 times the area of the principal peak in the chromatogram obtained with reference solution (a) (0.03 per cent).
Fluorides
Maximum 200 ppm.
Potentiometry (2.2.36, Method I). Prepare and store all solutions in plastic containers.
Buffer solution: Dissolve 58 g of sodium chloride R in 500 mL of water R. Add 57 mL of glacial acetic acid R and 200 mL of a 100 g/L solution of cyclohexylenedinitrilotetra-acetic acid R in 1 M sodium hydroxide. Adjust the pH to 5.0-5.5 with a 200 g/L solution of sodium hydroxide R and dilute to 1000.0 mL with water R.
Test solution: Dissolve 1.00 g of the substance to be examined in water R and dilute to 100.0 mL with the same solvent.
Reference solutions: Dissolve 4.42 g of sodium fluoride R, previously dried at 120 °C for 2 h, in 300 mL of water R and dilute to 1000.0 mL with the same solvent (solution (a): 1.9 g/L of fluoride). Prepare 3 reference solutions by dilution of solution (a) 1 in 100, 1 in 1000 and 1 in 10 000 respectively.
Indicator electrode: Fluoride selective.
Reference electrode: Silver-silver chloride.
To 20.0 mL of the test solution and each reference solution, add 10.0 mL of the buffer solution and stir with a magnetic stirrer. Introduce the electrodes into the solution and allow to stand for 5 min with constant stirring.
Calculate the concentration of fluorides using the calibration curve.
Loss on drying (2.2.32)
Maximum 1.0 per cent, determined on 1.000 g by drying in an oven at 105 °C.
Sulfated ash (2.4.14)
Maximum 0.1 per cent, determined on 1.0 g in a platinum crucible.
ASSAY
Dissolve 0.100 g in 40 mL of anhydrous acetic acid R and add 100 mL of acetic anhydride R. Titrate with 0.1 M perchloric acid determining the end-point potentiometrically (2.2.20).
1 mL of 0.1 M perchloric acid is equivalent to 12.91 mg of C4H4FN3O.
STORAGE
Protected from light.
IMPURITIES
Specified impurities A, B.
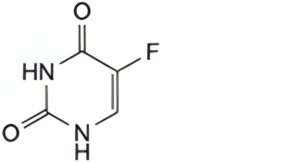
A. 5-fluoropyrimidine-2,4(1H,3H)-dione (fluorouracil),
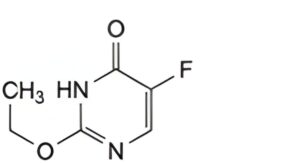
B. 2-ethoxy-5-fluoropyrimidin-4(3H)-one.