Edition: BP 2025 (Ph. Eur. 11.6 update)
Action and use
Dopamine receptor antagonist; neuroleptic.
DEFINITION
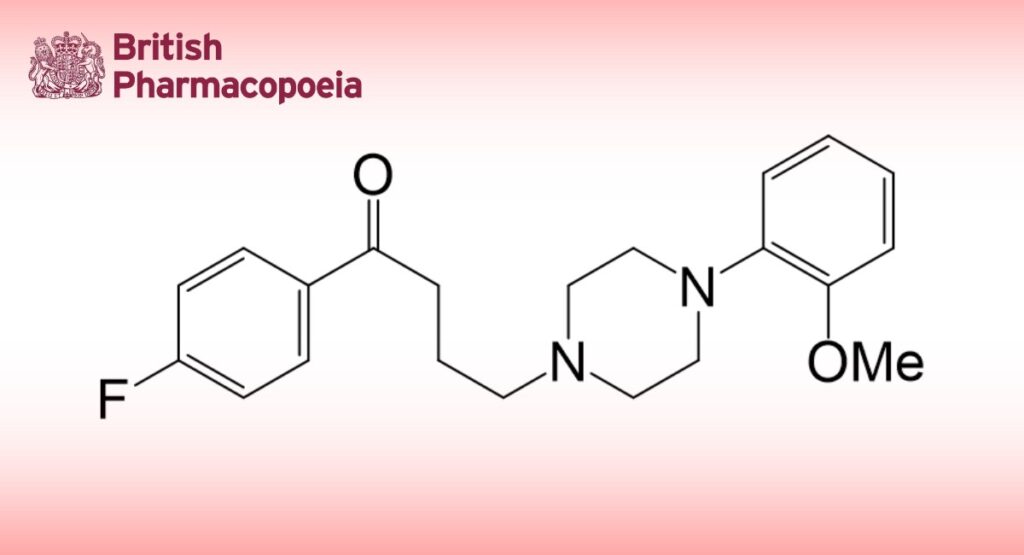
Fluanisone is 4′-fluoro-4-[4-(2-methoxyphenyl)piperazin-1-yl]-butyrophenone. It contains not less than 98.0% and not more than 101.0% of C21H25FN2O2, calculated with reference to the dried substance.
CHARACTERISTICS
White or almost white to buff-coloured crystals or powder; odourless or almost odourless. It exhibits polymorphism.
Practically insoluble in water; freely soluble in chloroform, in ethanol (96%), in ether and in dilute solutions of organic acids.
IDENTIFICATION
A. The infrared absorption spectrum, Appendix II A, is concordant with the reference spectrum of fluanisone (RSV 22). If the spectra are not concordant, dissolve 0.1 g of the substance being examined in 3 ml of dichloromethane and evaporate the solvent at room temperature, scratching the side of the container occasionally with a glass rod and prepare a new spectrum of the residue.
B. The light absorption, Appendix II B, in the range 230 to 350 nm of a 0.002% w/v solution in a mixture of 9 volumes of propan-2-ol and 1 volume of 0.1M hydrochloric acid exhibits a well-defined maximum only at 243 nm. The absorbance at 243 nm is about 1.1.
C. Heat 0.5 ml of chromic-sulphuric acid mixture in a small test tube in a water bath for 5 minutes; the solution wets the side of the tube readily and there is no greasiness. Add 2 to 3 mg of the substance being examined and again heat in a water bath for 5 minutes; the solution does not wet the side of the tube and does not pour easily from the tube.
TESTS
Melting point
72° to 76°, Appendix V A.
Related substances
Carry out the method for thin-layer chromatography, Appendix III A, using the following solutions.
(1) 2.0% w/v of the substance being examined.
(2) 0.010% w/v of the substance being examined.
(3) 0.020% w/v of 4′-fluoro-4-chlorobutyrophenone BPCRS.
(4) 0.010% w/v of 1-(2-methoxyphenyl)piperazine BPCRS.
CHROMATOGRAPHIC CONDITIONS
(a) Use as the coating silica gel GF254 precoated plate (Merck silica gel 60 plates are suitable).
(b) Use the mobile phase as described below.
(c) Apply 10 µl of each solution.
(d) Develop the plate to 15 cm.
(e) After removal of the plate, dry in air and expose to iodine vapour for 15 minutes.
MOBILE PHASE
10 volumes of ethanol (96%) and 90 volumes of chloroform.
LIMITS
In the chromatogram obtained with solution (1):
any spots corresponding to 4′-fluoro-4-chlorobutyrophenone and 1-(2-methoxyphenyl)piperazine are not more intense than the spots in the chromatograms obtained with solutions (3) and (4) respectively (1% and 0.5%, respectively);
any other secondary spot in the chromatogram obtained with solution (1) is not more intense than the spot in the chromatogram obtained with solution (2) (0.5%).
Loss on drying
When dried to constant weight at 40° at a pressure not exceeding 0.7 kPa, loses not more than 0.5% of its weight. Use 1 g.
Sulphated ash
Not more than 0.1%, Appendix IX A.
ASSAY
Carry out Method I for non-aqueous titration, Appendix VIII A, using 0.15 g and crystal violet solution as indicator. Each ml of 0.1M perchloric acid VS is equivalent to 17.82 mg of C21H25FN2O2.
STORAGE
Fluanisone should be protected from light.