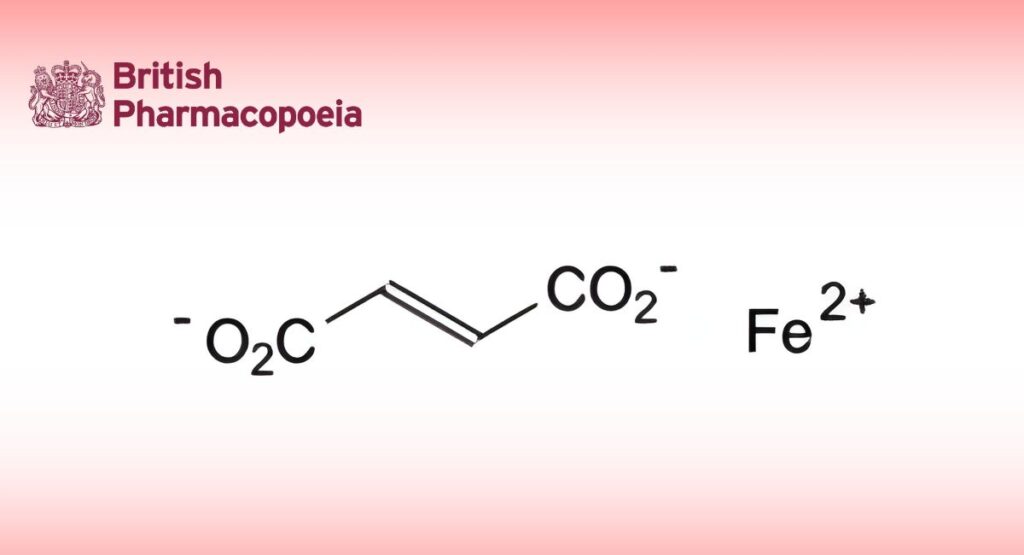
(Ph. Eur. monograph 0902)
C4H2FeO4 169.9 141-01-5
Action and use
Treatment of iron-deficiency anaemia.
Preparations
Ferrous Fumarate Capsules
Ferrous Fumarate Oral Suspension
Ferrous Fumarate Tablets
Ferrous Fumarate and Folic Acid Tablets
DEFINITION
Iron(II) (E)-butenedioate.
Content
93.0 per cent to 101.0 per cent (dried substance).
CHARACTERS
Appearance
Fine, reddish-orange or reddish-brown powder.
Solubility
Slightly soluble in water, very slightly soluble in ethanol (96 per cent).
IDENTIFICATION
A. Thin-layer chromatography (2.2.27).
Test solution To 1.0 g add 25 mL of a mixture of equal volumes of hydrochloric acid R and water R and heat on a water-bath for 15 min. Cool and filter. Use the filtrate for identification test C. Wash the residue with 50 mL of a mixture of 1 volume of dilute hydrochloric acid R and 9 volumes of water R and discard the washings. Dry the residue at 100-105 °C.
Dissolve 20 mg of the residue in acetone R and dilute to 10 mL with the same solvent.
Reference solution: Dissolve 20 mg of fumaric acid CRS in acetone R and dilute to 10 mL with the same solvent.
Plate TLC silica gel F254 plate R.
Mobile phase anhydrous formic acid R, methylene chloride R, butanol R, heptane R (12:16:32:44 V/V/V/V).
Application 5 μL.
Development: In an unsaturated tank, over a path of 10 cm.
Drying At 105 °C for 15 min.
Detection: Examine in ultraviolet light at 254 nm.
Results: The principal spot in the chromatogram obtained with the test solution is similar in position and size to the principal spot in the chromatogram obtained with the reference solution.
B. Mix 0.5 g with 1 g of resorcinol R. To 0.5 g of the mixture in a crucible add 0.15 mL of sulfuric acid R and heat gently. A dark red semi-solid mass is formed. Add the mass, with care, to 100 mL of water R. An orange-yellow colour develops and the solution shows no fluorescence.
C. The filtrate obtained during preparation of the test solution in identification test A gives reaction (a) of iron (2.3.1).
TESTS
Sulfates (2.4.13)
Maximum 0.2 per cent.
Heat 0.15 g with 8 mL of dilute hydrochloric acid R and 20 mL of distilled water R. Cool in iced water, filter and dilute to 30 mL with distilled water R.
Elemental impurities
Any method that fulfils the requirements of general chapter 2.4.20. Determination of elemental impurities may be used.
| Element | Maximum content (ppm) |
| Arsenic | 1.5 |
| Chromium | 200 |
| Cobalt | 20 |
| Lead | 3 |
| Nickel | 50 |
| Vanadium | 40 |
Ferric ion
Maximum 2.0 per cent.
In a flask with a ground-glass stopper, dissolve 3.0 g in a mixture of 10 mL of hydrochloric acid R and 100 mL of water R by heating rapidly to boiling. Boil for 15 s. Cool rapidly, add 3 g of potassium iodide R, stopper the flask and allow to stand protected from light for 15 min. Add 2 mL of starch solution R as indicator. Titrate the liberated iodine with 0.1 M sodium thiosulfate. Carry out a blank test. The difference between the volumes used in the 2 titrations corresponds to the amount of iodine liberated by ferric ion. 1 mL of 0.1 M sodium thiosulfate is equivalent to 5.585 mg of ferric ion.
Zinc
Maximum 500 ppm.
Atomic absorption spectrometry (2.2.23, Method I).
Test solution: Dissolve 0.500 g in a mixture of 2.5 mL of hydrochloric acid R and 20 mL of water R, heating slightly if necessary. Allow to cool, filter if necessary and dilute to 25.0 mL with water R. Dilute 1.0 mL of the solution to 10.0 mL with water R.
Reference solutions: Prepare the reference solutions using zinc standard solution (10 ppm Zn) R and diluting with a 1 per cent V/V solution of hydrochloric acid R.
Source Zinc hollow-cathode lamp.
Wavelength 213.9 nm.
Atomisation device Air-acetylene flame.
Loss on drying (2.2.32)
Maximum 1.0 per cent, determined on 1.000 g by drying in an oven at 105 °C.
ASSAY
Dissolve with slight heating 0.150 g in 7.5 mL of dilute sulfuric acid R. Cool and add 25 mL of water R. Add 0.1 mL of ferroin R. Titrate immediately with 0.1 M cerium sulfate until the colour changes from orange to light bluish-green.
1 mL of 0.1 M cerium sulfate is equivalent to 16.99 mg of C4H2FeO4.
STORAGE
In an airtight container, protected from light.