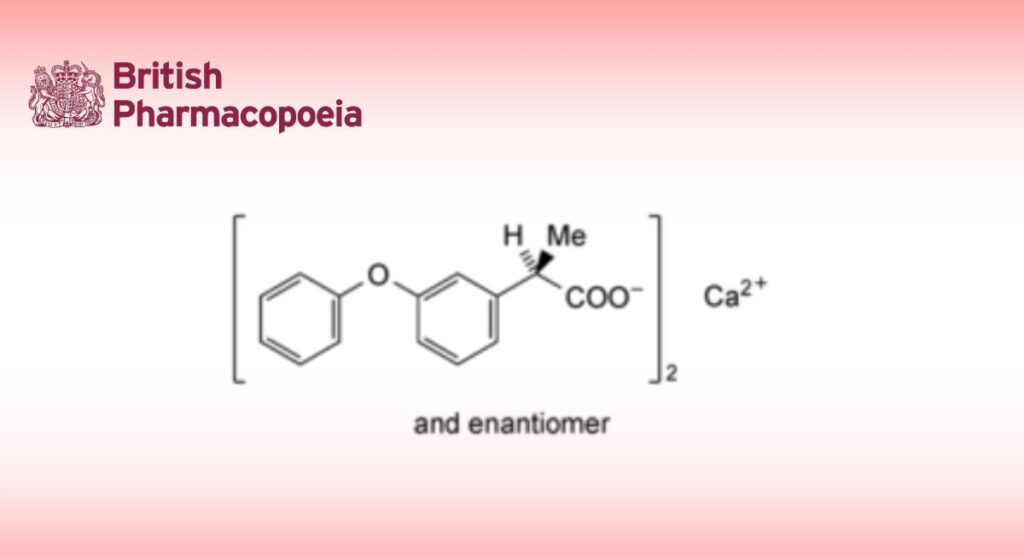
(C15H13O3)2Ca,2H2O 558.6 34957-40-5
Action and use
Cyclo-oxygenase inhibitor; analgesic; anti-inflammatory.
Preparation
Fenoprofen Tablets
DEFINITION
Fenoprofen Calcium is calcium (RS)-2-(3-phenoxyphenyl)propionate dihydrate. It contains not less than 97.5% and not more than 101.0% of (C15H13O3)2Ca, calculated with reference to the anhydrous substance.
CHARACTERISTICS
A white or almost white, crystalline powder.
Slightly soluble in water; soluble in ethanol (96%).
IDENTIFICATION
A. Dissolve 0.1 g in 5 mL of glacial acetic acid and add sufficient methanol to produce 100 mL. Dilute 5 mL of this solution to 50 mL with methanol. The light absorption of the resulting solution, Appendix II B, in the range 230 to 350 nm exhibits two maxima, at 272 nm and 278 nm, and a shoulder at 266 nm. The absorbance at the maximum at 272 nm is about 0.70 and at the maximum at 278 nm is about 0.65.
B. The infrared absorption spectrum, Appendix II A, is concordant with the reference spectrum of fenoprofen calcium (RS 142).
C. The residue on ignition yields the reactions characteristic of calcium salts, Appendix VI.
TESTS
Related substances
Carry out the method for liquid chromatography, Appendix III D, using the following solutions in mobile phase.
(1) 0.50% w/v of the substance being examined.
(2) 0.0025% w/v of the substance being examined.
(3) 0.04% w/v of fenoprofen calcium and 0.0015% w/v of 4,4′-dimethoxybenzophenone.
CHROMATOGRAPHIC CONDITIONS
(a) Use a stainless steel column (25 cm × 4.6 mm) packed with octadecylsilyl silica gel for chromatography (7 to 8 μm) (Zorbax ODS is suitable).
(b) Use isocratic elution and the mobile phase described below.
(c) Use a flow rate of 2 mL per minute.
(d) Use an ambient column temperature.
(e) Use a detection wavelength of 270 nm.
(f) Inject 20 μL of each solution.
(g) Allow the chromatography to proceed for 3 times the retention time of the peak due to fenoprofen.
MOBILE PHASE
2 volumes of glacial acetic acid, 7 volumes of tetrahydrofuran, 30 volumes of acetonitrile and 61 volumes of water.
SYSTEM SUITABILITY
The test is valid if the resolution factor between the peaks corresponding to fenoprofen and 4,4′- dimethoxybenzophenone in the chromatogram obtained with solution (3) is at least 3.0.
LIMITS
In the chromatogram obtained with solution (1):
the area of any secondary peak is not greater than twice the area of the peak in the chromatogram obtained with solution (2) (1%); not more than one secondary peak has an area greater than the area of the peak in the chromatogram obtained with solution (2) (0.5%); the sum of the areas of all secondary peaks is not greater than four times the area of the peak in the chromatogram obtained with solution (2) (2%).
Water
5.0 to 8.0% w/w, Appendix IX C. Use 0.2 g.
ASSAY
Carry out Method I for non-aqueous titration, Appendix VIII A, using 0.5 g and determining the end point potentiometrically.
Each mL of 0.1M perchloric acid VS is equivalent to 26.13 mg of (C15H13O3)2Ca.