(Ph. Eur. monograph 1634)
C46H65N13O11S2 1039 56-59-7
Action and use
Vasopressin analogue; vasoconstrictor in local anaesthesia.
DEFINITION
L-Cysteinyl-L-phenylalanyl-L-phenylalanyl-L-glutaminyl-L-asparaginyl-L-cysteinyl-L-prolyl-L-lysylglycinamide cyclic (1,6)-disulfide.
Synthetic nonapeptide having a vasoconstricting activity. It is available as an acetate.
Content
95.0 per cent to 102.0 per cent (anhydrous and acetic acid-free substance).
CHARACTERS
Appearance
White or almost white, powder or flakes.
Solubility
Freely soluble in water, practically insoluble in acetone and ethanol (96 per cent). It dissolves in dilute solutions of alkali hydroxides.
IDENTIFICATION
A. Examine the chromatograms obtained in the assay.
Results The principal peak in the chromatogram obtained with test solution (b) is similar in retention time and size to the principal peak in the chromatogram obtained with the reference solution.
B. Amino acid analysis (2.2.56). For hydrolysis use Method 1 and for analysis use Method 1.
Express the content of each amino acid in moles. Calculate the relative proportions of amino acids, taking one-seventh of the sum of the number of moles of glutamic acid, aspartic acid, proline, lysine, glycine and phenylalanine as equal to one.
The values fall within the following limits: aspartic acid: 0.9 to 1.1; glutamic acid: 0.9 to 1.1; proline: 0.9 to 1.1; glycine: 0.9 to 1.1; phenylalanine: 1.8 to 2.2; half-cystine: 1.8 to 2.2; lysine: 0.9 to 1.1.
TESTS
Specific optical rotation (2.2.7)
-35 to -29, determined at 25 °C (anhydrous and acetic acid-free substance).
Dissolve 20.0 mg in a 1 per cent V/V solution of glacial acetic acid R and dilute to 10.0 mL with the same solution.
Related substances
Liquid chromatography (2.2.29); use the normalisation procedure. The solutions are stable for 24 h at room temperature or for 1 week at 2-8 °C.
Test solution (a) Dissolve 5.0 mg of the substance to be examined in 5.0 mL of mobile phase A.
Test solution (b) Dilute 1.0 mL of test solution (a) to 5.0 mL with mobile phase A.
Reference solution Dissolve the contents of a vial of felypressin CRS in mobile phase A to obtain a concentration of 0.2 mg/mL.
Column:
— size: l = 0.15 m, Ø = 3.9 mm,
— stationary phase: octadecylsilyl silica gel for chromatography R (5 μm),
— temperature: 50 °C.
Mobile phase:
— mobile phase A: dissolve 3.62 g of tetramethylammonium hydroxide R in 900 mL water R; adjust to pH 2.5 with phosphoric acid R and dilute to 1000 mL with water R;
— mobile phase B: dissolve 1.81 g of tetramethylammonium hydroxide R in 450 mL of a 50 per cent V/V solution of acetonitrile for chromatography R; adjust to pH 2.5 with phosphoric acid R and dilute to 500 mL with a 50 per cent V/V solution of acetonitrile for chromatography R;
| Time (min) |
Mobile phase A (per cent V/V) |
Mobile phase B (per cent V/V) |
| 0 – 20 | 80 → 50 | 20 → 50 |
| 20 – 25 | 50 | 50 |
Flow rate 1.0 mL/min.
Detection Spectrophotometer at 210 nm.
Injection 10 μL of test solution (a) and 50 μL of the reference solution.
Identification of impurities: Use the chromatogram supplied with felypressin CRS to identify the peaks due to impurities A to F.
Relative retention With reference to felypressin: impurity A = about 0.9; impurity B = about 1.1; impurity F = about 1.2; impurity C = about 1.3; impurity D = about 1.4; impurity E = about 2.1.
System suitability Reference solution:
— retention time: felypressin = about 7.5 min;
— resolution: minimum 1.5 between the peaks due to impurity C and impurity D.
Limits:
— impurities A, B, C, D, E, F: for each impurity, maximum 0.5 per cent,
— any other impurity: for each impurity, maximum 0.1 per cent,
— total: maximum 3.0 per cent,
— disregard limit: 0.05 per cent.
Acetic acid (2.5.34)
9.0 per cent to 13.0 per cent.
Test solution Dissolve 10.0 mg of the substance to be examined in a mixture of 5 volumes of mobile phase B and 95 volumes of mobile phase A and dilute to 10.0 mL with the same mixture of mobile phases.
Water (2.5.32)
Maximum 7.0 per cent.
Bacterial endotoxins (2.6.14)
Less than 100 IU/mg, if intended for use in the manufacture of parenteral preparations without a further appropriate procedure for the removal of bacterial endotoxins.
ASSAY
Liquid chromatography (2.2.29) as described in the test for related substances with the following modification.
Injection 10 μL of test solution (b) and of the reference solution.
Calculate the content of felypressin (C46H65N13O11S2) from the areas of the peaks and the declared content of C46H65N13O11S2 in felypressin CRS.
STORAGE
In an airtight container, protected from light, at a temperature of 2 °C to 8 °C. If the substance is sterile, store in a sterile, airtight, tamper-evident container.
LABELLING
The label states the mass of peptide in the container.
IMPURITIES
Specified impurities A, B, C, D, E, F.
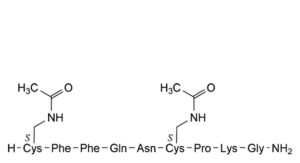
A. S ,S -bis[(acetylamino)methyl]-(reduced felypressin), 1 6
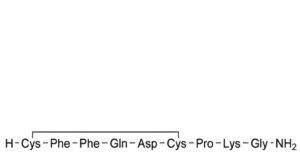
B. [5-aspartic acid]felypressin,

C. bis(reduced felypressin) (1,6′),(1′,6)-bis(disulfide),

D. bis(reduced felypressin) (1,1′),(6,6′)-bis(disulfide),
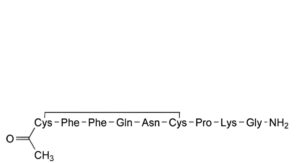
E. N -acetylfelypressin,
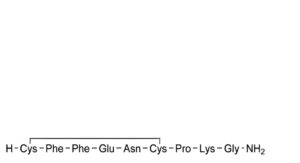
F. [4-glutamic acid]felypressin.






